User login
LOS ANGELES – An investigational type of photodynamic therapy appears promising for treating early cervical intraepithelial neoplasia, finds a phase 2b trial reported at the annual meeting of the Society of Gynecologic Oncology.
A team led by Dr. Mark H. Einstein undertook a dose-finding study of hexaminolevulinate (HAL) gel coupled with photodynamic therapy in 262 patients with grade 1 or 2 cervical intraepithelial neoplasia (CIN1, CIN2).
Among patients with CIN2, nearly all of those treated with the highest dose of HAL and photodynamic therapy had a response at 3 months, compared with roughly half of their counterparts treated with placebo.
The therapy was associated with a higher rate of local reactions, but none had to discontinue treatment.
"The 5% HAL photodynamic therapy was effective in treating patients with CIN2. There seems to be a signal of efficacy in patients who have HPV 16 and 18 as well," which may help inform the design of future phase III trials, commented Dr. Einstein, who is director of clinical research for women’s health and gynecologic oncology at the Montefiore Medical Center, New York.
"This device was very easy to use and was very well tolerated by this patient population, with essentially self-limiting reactions," he added.
Dr. Einstein noted that new approaches are needed for dealing with CIN, given that it commonly occurs in young women of childbearing age, and that some current treatments aimed at obliterating these lesions make subsequent colposcopies difficult and increase the risk of preterm birth.
For photodynamic therapy, a photosensitizing agent is applied to the cervix and accumulates in the premalignant cells, he explained. When the area is illuminated at a specific energy, reactive oxygen species are generated, killing the cells.
"The hypothesized mechanism of photodynamic therapy in cervical lesions is that once the HAL is applied and light is emitted for about 5 hours, this induces some local apoptosis, and that will release a lot of HPV antigens from these dying cells. These are then presented to naive T cells in the locoregional lymph nodes, which then stimulates even more of a cell-mediated immune response and inflammatory response that then further increases apoptosis of local cells," Dr. Einstein elaborated. "And then, hypothetically, in other areas of the cervical and vaginal tract, you are going to get a big cell-mediated immune response."
Women in the trial, which was sponsored by Photocure, had pathologically confirmed, previously untreated CIN1 or CIN2.
They were randomized evenly to four groups treated with HAL 5%, 1%, or 0.2%, or placebo, and photodynamic therapy.
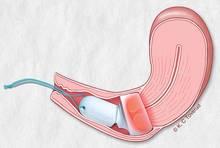
A gynecologist placed the integrated drug and device (Cevira, manufactured by Photocure) in the outpatient setting. Five hours after placement, the device self-activated and delivered photodynamic therapy for roughly 5 hours. The patients removed the device themselves thereafter.
The patients had a median age of 27 years, and 46% had CIN2. About half of those tested were found to have a high-risk type of HPV.
Among patients with CIN2, 5% HAL photodynamic therapy yielded a higher 3-month rate of response (based on histology, cytology, and HPV status) than did placebo, both overall (95% vs. 56%; P = .01) and in the subset who were positive for any type of HPV (92% vs. 50%; P = .02).
"Clearly, the placebo effect was very large in this group, and this is expected in young women who have CIN1 and CIN2. That being said, this was significant efficacy when compared to placebo," said Dr. Einstein, who disclosed that his institution has received funding from Photocure for educational speaking activities and research.
Additionally, among the patients with CIN2 who had HPV 16 or 18 infection, those treated with 5% HAL photodynamic therapy were more likely than were those treated with placebo to have clearance of the virus (83% vs. 0%; P = .02).
There was no significant benefit among women with CIN1 or among women treated with the lower doses of HAL.
Relative to their counterparts treated with placebo, patients treated with 5% HAL photodynamic therapy had a higher rate of self-limiting local reactions (54% vs. 32%); the most common were vaginal discharge and local discomfort. None of the patients discontinued treatment because of these reactions.
A total of five pregnancies were reported among study patients in the 3 months after treatment. All of these women delivered normal full-term infants.
LOS ANGELES – An investigational type of photodynamic therapy appears promising for treating early cervical intraepithelial neoplasia, finds a phase 2b trial reported at the annual meeting of the Society of Gynecologic Oncology.
A team led by Dr. Mark H. Einstein undertook a dose-finding study of hexaminolevulinate (HAL) gel coupled with photodynamic therapy in 262 patients with grade 1 or 2 cervical intraepithelial neoplasia (CIN1, CIN2).
Among patients with CIN2, nearly all of those treated with the highest dose of HAL and photodynamic therapy had a response at 3 months, compared with roughly half of their counterparts treated with placebo.
The therapy was associated with a higher rate of local reactions, but none had to discontinue treatment.
"The 5% HAL photodynamic therapy was effective in treating patients with CIN2. There seems to be a signal of efficacy in patients who have HPV 16 and 18 as well," which may help inform the design of future phase III trials, commented Dr. Einstein, who is director of clinical research for women’s health and gynecologic oncology at the Montefiore Medical Center, New York.
"This device was very easy to use and was very well tolerated by this patient population, with essentially self-limiting reactions," he added.
Dr. Einstein noted that new approaches are needed for dealing with CIN, given that it commonly occurs in young women of childbearing age, and that some current treatments aimed at obliterating these lesions make subsequent colposcopies difficult and increase the risk of preterm birth.
For photodynamic therapy, a photosensitizing agent is applied to the cervix and accumulates in the premalignant cells, he explained. When the area is illuminated at a specific energy, reactive oxygen species are generated, killing the cells.
"The hypothesized mechanism of photodynamic therapy in cervical lesions is that once the HAL is applied and light is emitted for about 5 hours, this induces some local apoptosis, and that will release a lot of HPV antigens from these dying cells. These are then presented to naive T cells in the locoregional lymph nodes, which then stimulates even more of a cell-mediated immune response and inflammatory response that then further increases apoptosis of local cells," Dr. Einstein elaborated. "And then, hypothetically, in other areas of the cervical and vaginal tract, you are going to get a big cell-mediated immune response."
Women in the trial, which was sponsored by Photocure, had pathologically confirmed, previously untreated CIN1 or CIN2.
They were randomized evenly to four groups treated with HAL 5%, 1%, or 0.2%, or placebo, and photodynamic therapy.
A gynecologist placed the integrated drug and device (Cevira, manufactured by Photocure) in the outpatient setting. Five hours after placement, the device self-activated and delivered photodynamic therapy for roughly 5 hours. The patients removed the device themselves thereafter.
The patients had a median age of 27 years, and 46% had CIN2. About half of those tested were found to have a high-risk type of HPV.
Among patients with CIN2, 5% HAL photodynamic therapy yielded a higher 3-month rate of response (based on histology, cytology, and HPV status) than did placebo, both overall (95% vs. 56%; P = .01) and in the subset who were positive for any type of HPV (92% vs. 50%; P = .02).
"Clearly, the placebo effect was very large in this group, and this is expected in young women who have CIN1 and CIN2. That being said, this was significant efficacy when compared to placebo," said Dr. Einstein, who disclosed that his institution has received funding from Photocure for educational speaking activities and research.
Additionally, among the patients with CIN2 who had HPV 16 or 18 infection, those treated with 5% HAL photodynamic therapy were more likely than were those treated with placebo to have clearance of the virus (83% vs. 0%; P = .02).
There was no significant benefit among women with CIN1 or among women treated with the lower doses of HAL.
Relative to their counterparts treated with placebo, patients treated with 5% HAL photodynamic therapy had a higher rate of self-limiting local reactions (54% vs. 32%); the most common were vaginal discharge and local discomfort. None of the patients discontinued treatment because of these reactions.
A total of five pregnancies were reported among study patients in the 3 months after treatment. All of these women delivered normal full-term infants.
LOS ANGELES – An investigational type of photodynamic therapy appears promising for treating early cervical intraepithelial neoplasia, finds a phase 2b trial reported at the annual meeting of the Society of Gynecologic Oncology.
A team led by Dr. Mark H. Einstein undertook a dose-finding study of hexaminolevulinate (HAL) gel coupled with photodynamic therapy in 262 patients with grade 1 or 2 cervical intraepithelial neoplasia (CIN1, CIN2).
Among patients with CIN2, nearly all of those treated with the highest dose of HAL and photodynamic therapy had a response at 3 months, compared with roughly half of their counterparts treated with placebo.
The therapy was associated with a higher rate of local reactions, but none had to discontinue treatment.
"The 5% HAL photodynamic therapy was effective in treating patients with CIN2. There seems to be a signal of efficacy in patients who have HPV 16 and 18 as well," which may help inform the design of future phase III trials, commented Dr. Einstein, who is director of clinical research for women’s health and gynecologic oncology at the Montefiore Medical Center, New York.
"This device was very easy to use and was very well tolerated by this patient population, with essentially self-limiting reactions," he added.
Dr. Einstein noted that new approaches are needed for dealing with CIN, given that it commonly occurs in young women of childbearing age, and that some current treatments aimed at obliterating these lesions make subsequent colposcopies difficult and increase the risk of preterm birth.
For photodynamic therapy, a photosensitizing agent is applied to the cervix and accumulates in the premalignant cells, he explained. When the area is illuminated at a specific energy, reactive oxygen species are generated, killing the cells.
"The hypothesized mechanism of photodynamic therapy in cervical lesions is that once the HAL is applied and light is emitted for about 5 hours, this induces some local apoptosis, and that will release a lot of HPV antigens from these dying cells. These are then presented to naive T cells in the locoregional lymph nodes, which then stimulates even more of a cell-mediated immune response and inflammatory response that then further increases apoptosis of local cells," Dr. Einstein elaborated. "And then, hypothetically, in other areas of the cervical and vaginal tract, you are going to get a big cell-mediated immune response."
Women in the trial, which was sponsored by Photocure, had pathologically confirmed, previously untreated CIN1 or CIN2.
They were randomized evenly to four groups treated with HAL 5%, 1%, or 0.2%, or placebo, and photodynamic therapy.
A gynecologist placed the integrated drug and device (Cevira, manufactured by Photocure) in the outpatient setting. Five hours after placement, the device self-activated and delivered photodynamic therapy for roughly 5 hours. The patients removed the device themselves thereafter.
The patients had a median age of 27 years, and 46% had CIN2. About half of those tested were found to have a high-risk type of HPV.
Among patients with CIN2, 5% HAL photodynamic therapy yielded a higher 3-month rate of response (based on histology, cytology, and HPV status) than did placebo, both overall (95% vs. 56%; P = .01) and in the subset who were positive for any type of HPV (92% vs. 50%; P = .02).
"Clearly, the placebo effect was very large in this group, and this is expected in young women who have CIN1 and CIN2. That being said, this was significant efficacy when compared to placebo," said Dr. Einstein, who disclosed that his institution has received funding from Photocure for educational speaking activities and research.
Additionally, among the patients with CIN2 who had HPV 16 or 18 infection, those treated with 5% HAL photodynamic therapy were more likely than were those treated with placebo to have clearance of the virus (83% vs. 0%; P = .02).
There was no significant benefit among women with CIN1 or among women treated with the lower doses of HAL.
Relative to their counterparts treated with placebo, patients treated with 5% HAL photodynamic therapy had a higher rate of self-limiting local reactions (54% vs. 32%); the most common were vaginal discharge and local discomfort. None of the patients discontinued treatment because of these reactions.
A total of five pregnancies were reported among study patients in the 3 months after treatment. All of these women delivered normal full-term infants.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major finding: Patients with CIN2 were significantly more likely to have a response when treated with the highest dose of HAL photodynamic therapy than when treated with placebo (95% vs. 56%).
Data source: A randomized double-blind phase 2b trial of HAL photodynamic therapy in 262 patients with CIN1 or CIN2
Disclosures: Dr. Einstein disclosed that his institution has received funding from Photocure for educational speaking activities and research. The trial was sponsored by Photocure.