User login
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
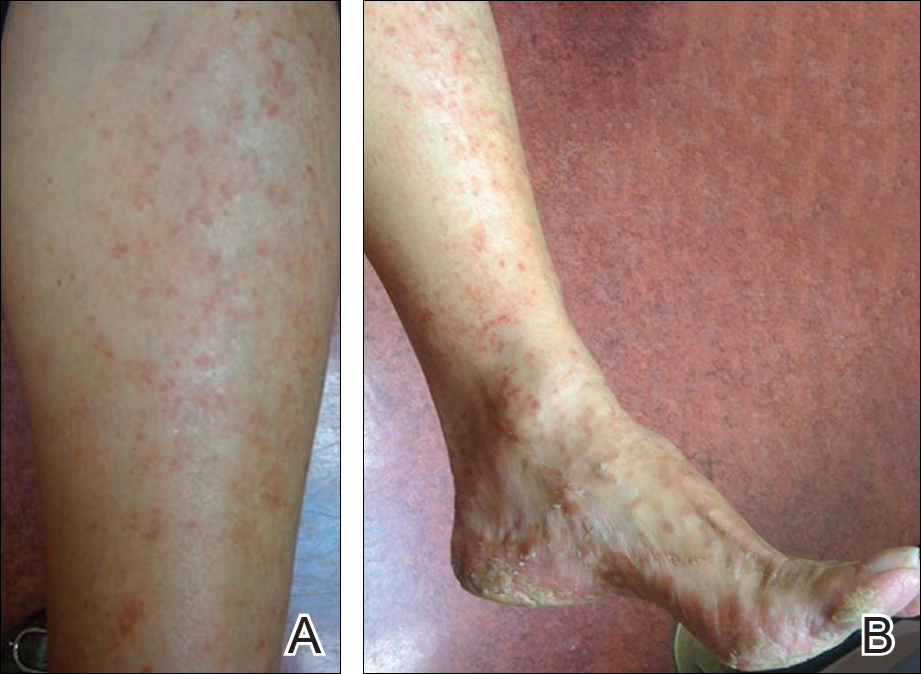
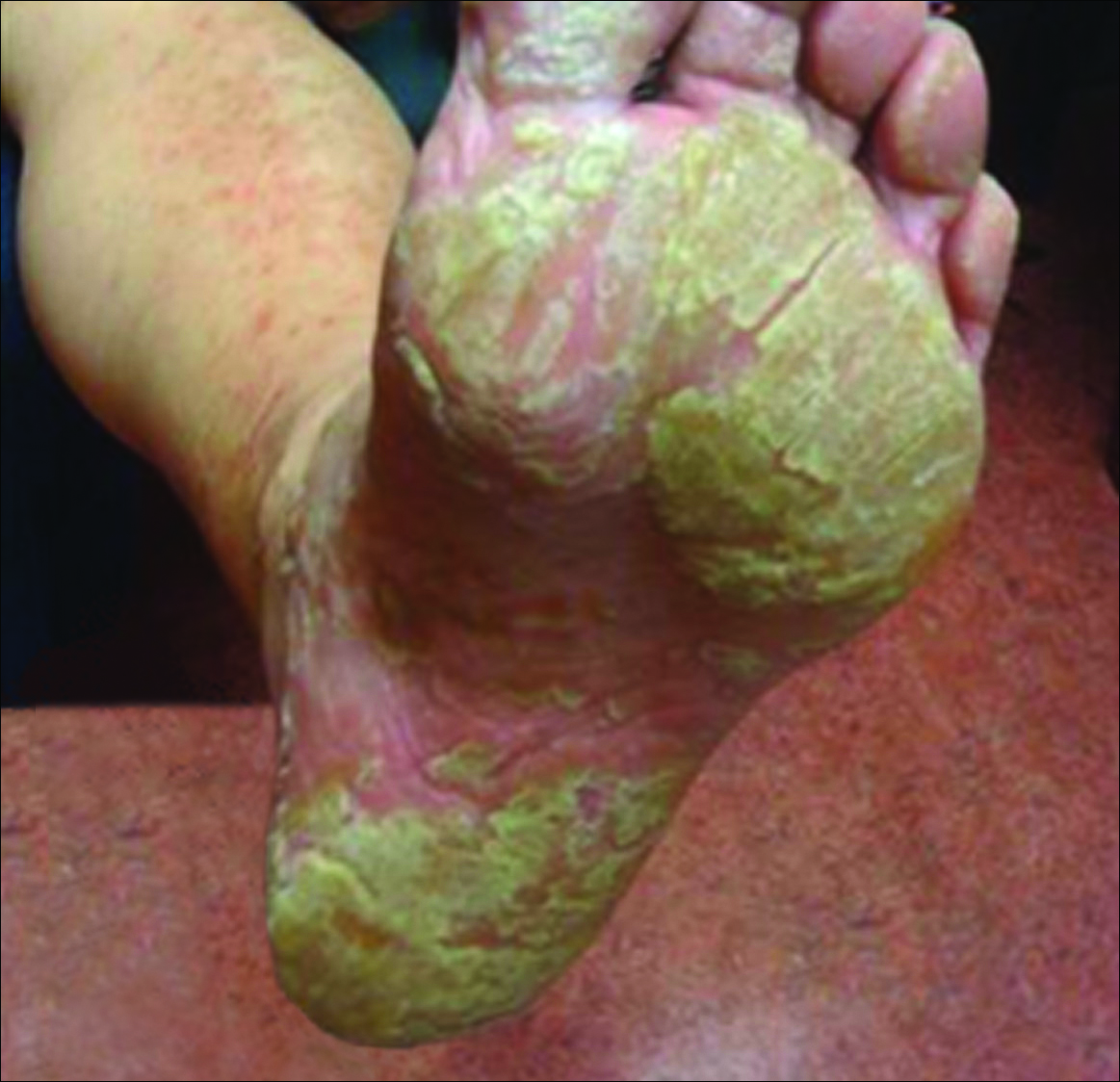
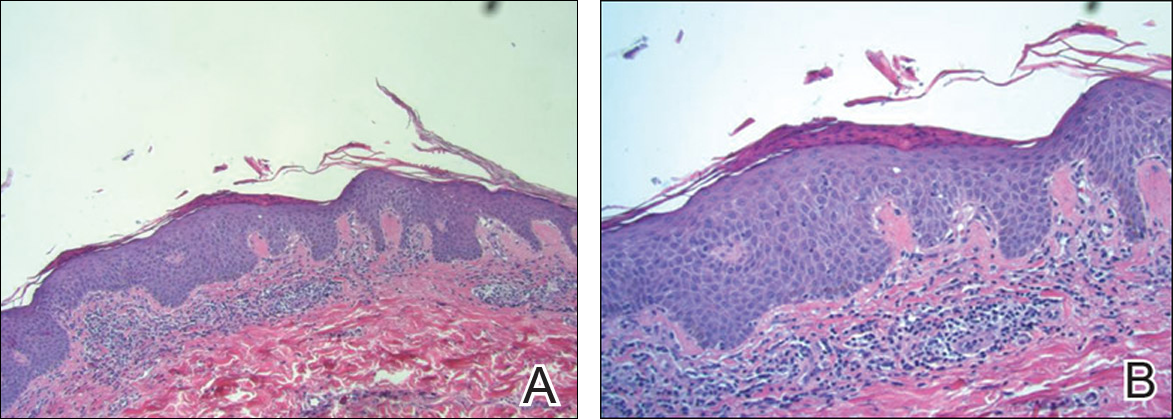
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).
The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Practice Points
- Diagnosis of pityriasis lichenoides may be difficult due to a wide spectrum of clinical presentations.
- Pityriasis lichenoides chronica (PLC) with palmoplantar involvement may mimic psoriasis.
- Screening for infections is not recommended in patients with PLC unless the patient has other symptoms pointing to a specific infection.
- Phototherapy currently is the most effective treatment modality for PLC.