User login
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.
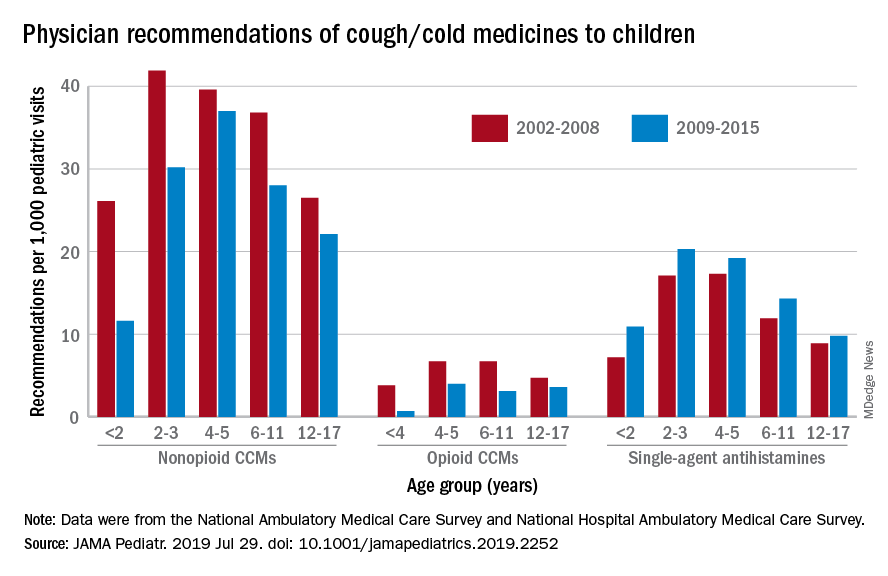
Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
FROM JAMA PEDIATRICS