User login
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
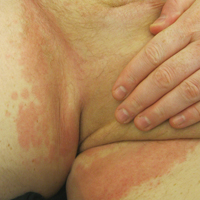
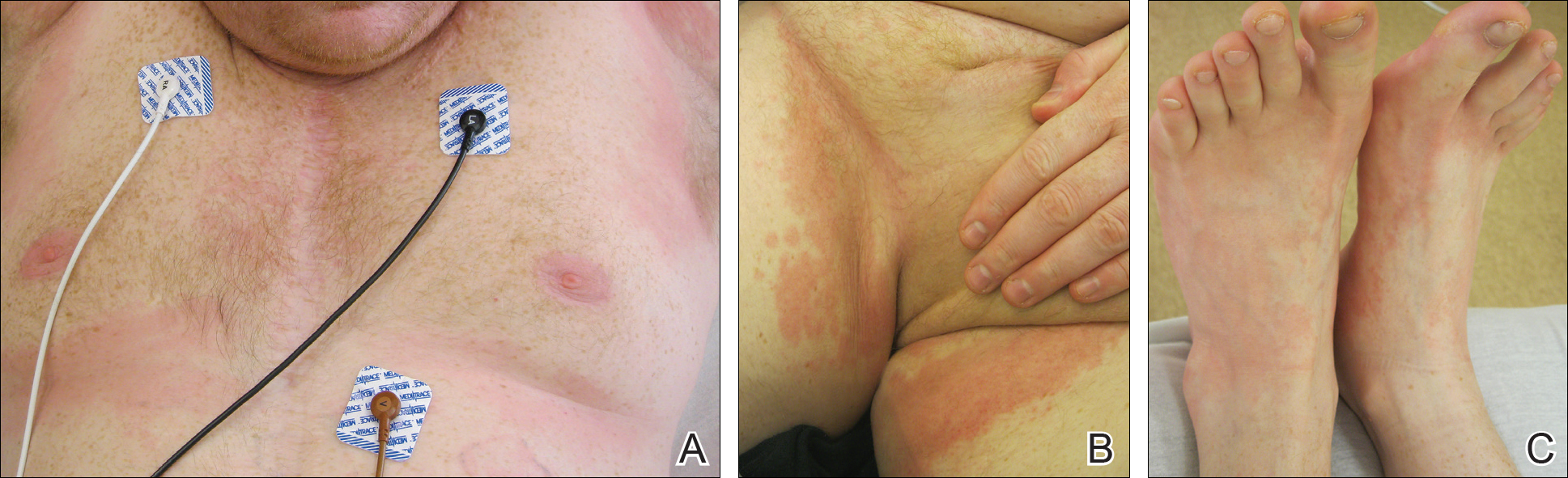
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.
Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.

Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
Serum sickness was first described by von Pirquet and Schick1 as a constellation of signs and symptoms displayed in patients receiving equine serum as an antitoxin for the treatment of scarlet fever and diphtheria. Serum sickness is an immune complex–mediated hypersensitivity reaction that can be clinically diagnosed in patients who present with fever, rash, and polyarthralgia or polyarthritis following exposure to heterologous serum proteins.2,3 Symptom onset typically occurs within 1 to 2 weeks of first exposure to the serum, and resolution frequently occurs with discontinuation of the offending agent. Other symptoms may include malaise, gastrointestinal tract concerns, headache, blurred vision, or lymphadenopathy.4 Proteinuria, hematuria, and a transient decrease in creatinine clearance also have been reported in serum sickness.4
Serum sickness is caused by a type III immune complex–mediated hypersensitivity reaction to heterologous rabbit or equine serum proteins. Nonhuman proteins present in antithymocyte globulin (ATG) stimulate the production of IgG, IgM, IgA, and IgE antibodies.2-4 If the resultant immune complexes overwhelm the mononuclear phagocyte system, these complexes are deposited in blood vessels and tissues, which leads to complement activation and the production of complement fragments such as C3a and C5a.5 C3a is an anaphylatoxin that causes mast cell degranulation and the consequent formation of urticarial lesions. C5a is a neutrophil chemoattractant that promotes inflammation at the site of complement deposition.
Serum sickness–like reactions may occur days to weeks following administration of certain drugs, such as cefaclor or penicillin. Although the symptoms and timing of serum sickness–like reactions are similar to serum sickness, they are not caused by an immune complex–mediated mechanism and are believed to be secondary to an idiosyncratic delayed drug reaction.6
Thymoglobulin, a type of ATG, is a polyclonal antibody generated in rabbits that targets numerous human epitopes, including cell surface markers on T cells (CD2, CD3, CD4, CD8), B cells (CD21, CD19, CD40), and adhesion molecules (CD6, CD25, CD44, CD45, and the integrin LFA-1 [lymphocyte function-associated antigen-1]).7,8 Thymoglobulin has proven efficacy in the setting of cardiac transplantation.9-11 Although calcineurin inhibitors form the foundation in the armamentarium of immunosuppressive agents in cardiac transplantation, their nephrotoxicity has limited their unrestrained use in patients.9 By delaying the need for calcineurin inhibitors, thymoglobulin preserves greater renal function without increasing the risk for acute rejection.9,10 Akin to its use in the patient presented in this case report, thymoglobulin also is used in the treatment of acute cellular rejection in heart transplant recipients with signs of heart failure.11
Case Report
A 35-year-old man with a history of familial cardiomyopathy who underwent orthotopic heart transplantation presented with grade 3R acute cellular rejection. The patient’s immunosuppressive regimen consisted of thymoglobulin 150 mg once daily, tacrolimus 2.5 mg twice daily, hydrocortisone 100 mg once daily, and mycophenolate mofetil 1000 mg twice daily. On day 7 of thymoglobulin treatment, the dermatology department was consulted to evaluate a pruritic eruption. The patient reported that he noticed redness of the palms and soles, as well as redness accentuated in the axilla, groin, and other skin creases 2 days prior. The patient also reported symmetric bilateral hand pain that had started 1 day following rash onset. He denied fever and remained afebrile throughout his hospitalization.
On physical examination, the patient displayed a blanching, erythematous, edematous, evanescent macular rash with some areas of wheal formation symmetrically distributed in the bilateral axillae, inframammary folds, and groin (Figure, A and B). The palms and soles were tender with diffuse blanching erythema. The eruption was accentuated at the lateral and medial borders of both feet (Figure, C). There was concern that the patient may have a form of serum sickness with a blunted incomplete response due to his concomitant use of immunosuppressive agents. Shortly after evaluation, the patient left the hospital against medical advice before the recommended evaluation and systemic workup could be implemented.
The patient returned for an outpatient appointment approximately 1 week later. Medical records indicated that the patient’s skin eruption had resolved. Tests for antithymoglobulin antibodies at this visit were negative. The antithymoglobulin antibody enzyme-linked immunosorbent assay has a diagnostic sensitivity of 86%12 and large interlaboratory variability.13 Given the presence of other features of serum sickness, a false-negative result was considered by dermatology. Nonetheless, one must consider other differential diagnoses, including a simple cutaneous adverse drug eruption or viral exanthem that might have in fact been causative.

Comment
We present an atypical case of possible serum sickness in a heart transplant recipient following thymoglobulin treatment of acute cellular rejection of the cardiac allograft. Serum sickness is a clinical diagnosis supported by laboratory data. Some authors have suggested major and minor diagnostic criteria to aid with the diagnosis.7 Major diagnostic criteria include onset more than 7 days after the initial thymoglobulin administration, persistent high fevers (temperature, >38.4°C), persistent arthritis/arthralgia, and positive heterologous antibodies on enzyme-linked immunosorbent assay. Minor diagnostic criteria include rash, acute renal failure, trismus, and low serum complement (C3 and C4).
The variable cutaneous presentations of serum sickness are important to recognize in the process of making the correct diagnosis. Rash is frequently reported in serum sickness, with some studies displaying rates of up to 93%.4,14 The skin findings are most frequently described as urticarial or serpiginous macular lesions.3 Other variations of the eruption exist, and morbilliform eruptions or a combination of morbilliform and urticarial eruptions have been reported.3 It is important to judge cutaneous eruptions of serum sickness within the context of the potential cytopenia in a patient being treated with ATG. As such, purpuric eruptions have been attributed to serum sickness in thrombocytopenic patients receiving ATG for bone marrow failure.14
Usually, cutaneous eruptions of serum sickness initially are identified in the groin, axilla, and periumbilical region, and then they proceed to include the trunk and extremities. Erythema of the palms and soles frequently is described as well as a linear accentuation of the rash along the lateral and medial borders of the feet and hands at the margin of the plantar or palmar skin, respectively.14 The mucous membranes frequently are spared in serum sickness.
Despite the lack of evidence-based guidelines, case series and literature reviews have suggested a treatment regimen for serum sickness,7,15-18 calling for immediate withdrawal of the offending agent. Antihistamines may be added to control pruritus and rash. Patients with high fever, a progressive rash, or severe arthralgia have benefited from short courses of oral16,18 or intravenous7,17 glucocorticoids. The extent of the eruption in our patient was concerning, particularly because he was already receiving systemic corticosteroids in conjunction with other immunosuppressives, which may have explained his lack of fever.
Because our patient satisfied some diagnostic criteria for serum sickness and failed to satisfy others, our team was faced with the challenge of balancing the risks of possible serum sickness with the risks of the potential for progressive cardiac rejection from the withdrawal of thymoglobulin.7 There is some evidence in the literature for the use of therapeutic plasma exchange (TPE) for the treatment of serum sickness if the offending agent could not be discontinued. Tanriover et al19 presented a case series of 5 renal transplant recipients treated with thymoglobulin who developed serum sickness. The diagnosis of serum sickness was made clinically and augmented by the presence of antiheterologous antibodies. All 5 patients had persistent symptoms of serum sickness despite 2 days of glucocorticoid treatment. Interestingly, 3 patients had complete resolution of all symptoms after a single TPE treatment, and 2 patients achieved resolution of fever and arthritis after 2 consecutive days of TPE treatments.19 Because plasmapheresis is used to treat cardiac allograft rejection in patients showing signs of heart failure,11 the employment of TPE in these patients may have dual beneficial effects of concurrently treating serum sickness and allograft rejection.
Given the patient’s noncompliance and leaving the hospital against medical advice, a full workup was not able to be pursued in this case, though fortunately the eruption and his other symptoms had resolved by the time he was seen for outpatient follow-up 1 week later. Noncompliance with immunosuppressive therapy is a considerable risk factor for morbidity and mortality following heart transplantation. These patients have more transplant coronary artery disease and substantially shorter clinical event-free time.20 Our patient demonstrates the need for proactive compliance-enhancing interventions in heart transplant patients who experience allograft rejection.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
- von Pirquet C, Schick B. Serum Sickness. Schick B, trans-ed. Baltimore, MD; Williams & Wilkins; 1951.
- Vincent C, Revillard JP. Antibody response to horse gamma-globulin in recipients of renal allografts: relationship with transplant crises and transplant survival. Transplantation. 1977;24:141-147.
- Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407-1413.
- Bielory L, Gascon P, Lawley TJ, et al. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988;67:40-57.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286.
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587-1591.
- Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650.
- Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194-1200.
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22:78-89.
- Cantarovich M, Giannetti N, Barkun J, et al. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779-781.
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2010;9:160-167.
- Tatum AH, Bollinger RR, Sanfilippo F. Rapid serologic diagnosis of serum sickness from antithymocyte globulin therapy using enzyme immunoassay. Transplantation. 1984;38:582-586.
- Kimball JA, Pescovitz MD, Book BK, et al. Reduced human IgG anti-ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation. 1995;60:1379-1383.
- Bielory L, Yancey KB, Young NS, et al. Cutaneous manifestations of serum sickness in patients receiving antithymocyte globulin. J Am Acad Dermatol. 1985;13:411-417.
- Joubert GI, Hadad K, Matsui D, et al. Selection of treatment of cefaclor-associated urticarial, serum sickness-like reactions and erythema multiforme by emergency pediatricians: lack of a uniform standard of care. Can J Clin Pharmacol. 1999;6:197-201.
- Clark BM, Kotti GH, Shah AD, et al. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26:705-708.
- Finger E, Scheinberg M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J Clin Rheumatol. 2007;13:94-95.
- Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86:330-334.
- Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody-induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279-281.
- Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. 2004;23:1245-1251.
Practice Points
- Serum sickness can be seen in patients treated with thymoglobulin to prevent transplant rejection.
- Serum sickness can display multiple cutaneous manifestation, thus making it an important entity for dermatologists.