User login
Orthostatic hypotension is a chronic, debilitating illness associated with common neurologic conditions (eg, diabetic neuropathy, Parkinson disease). It is common in the elderly, especially in those who are institutionalized and are using multiple medications.
Treatment can be challenging, especially if the problem is neurogenic. This condition has no cure, symptoms vary in different circumstances, treatment is nonspecific, and aggressive treatment can lead to marked supine hypertension.
This review focuses on the prevention and treatment of neurogenic causes of orthostatic hypotension. We emphasize a simple but effective patient-oriented approach to management, using a combination of nonpharmacologic strategies and drugs clinically proven to be efficacious. The recommendations and their rationale are organized in a practical and easy-to-remember format for both physicians and patients.
WHAT HAPPENS WHEN WE STAND UP?
When we stand up, the blood goes down from the chest to the distensible venous capacitance system below the diaphragm. This fluid shift produces a decrease in venous return, ventricular filling, cardiac output, and blood pressure.1
This gravity-induced drop in blood pressure, detected by arterial baroreceptors in the aortic arch and carotid sinus, triggers a compensatory reflex tachycardia and vasoconstriction that restores normotension in the upright position. This compensatory mechanism is termed a baroreflex; it is mediated by afferent and efferent autonomic peripheral nerves and is integrated in autonomic centers in the brainstem.2
Orthostatic hypotension is the result of baroreflex failure (autonomic failure), end-organ dysfunction, or volume depletion. Injury to any limb of the baroreflex causes neurogenic orthostatic hypotension, although with afferent lesions alone, the hypotension tends to be modest and accompanied by wide fluctuations in blood pressure, including severe hypertension. Drugs can produce orthostatic hypotension by interfering with the autonomic pathways or their target end-organs or by affecting intravascular volume. Brain hypoperfusion, resulting from orthostatic hypotension from any cause, can lead to symptoms of orthostatic intolerance (eg, lightheadedness) and falls, and if the hypotension is severe, to syncope.
A DECREASE OF 20 MM HG SYSTOLIC OR 10 MM HG DIASTOLIC
The consensus definition of orthostatic hypotension is a reduction of systolic blood pressure of at least 20 mm Hg or a reduction of diastolic blood pressure of at least 10 mm Hg within 3 minutes of erect standing.3 A transient drop that occurs with abrupt standing and resolves rapidly suggests a benign condition, such as dehydration, rather than autonomic failure.
In the laboratory, patients are placed on a tilt table in the head-up position at an angle of at least 60 degrees to detect orthostatic changes in blood pressure. In the office, 1 minute of standing probably detects nearly all cases of orthostatic hypotension; however, standing beyond 2 minutes helps establish the severity (a further drop in blood pressure).4 Orthostatic hypotension developing after 3 minutes of standing is uncommon and may represent a reflex presyncope (eg, vasovagal) or a mild or early form of sympathetic adrenergic dysfunction.4, 5
NEUROGENIC AND NONNEUROGENIC CAUSES
Orthostatic hypotension may result from neurogenic and nonneurogenic causes.
Neurogenic orthostatic hypotension can be due to neuropathy (eg, diabetic or autoimmune neuropathies) or to central lesions (eg, Parkinson disease or multiple system atrophy). Its presence, severity, and temporal course can be important clues in diagnosing Parkinson disease and differentiating it from other parkinsonian syndromes with a more ominous prognosis, such as multiple system atrophy and Lewy body dementia.
Nonneurogenic causes include cardiac impairment (eg, from myocardial infarction or aortic stenosis), reduced intravascular volume (eg, from dehydration, adrenal insufficiency), and vasodilation (eg, from fever, systemic mastocytosis).
Common drugs that cause orthostatic hypotension are diuretics, alpha-adrenoceptor blockers for prostatic hypertrophy, antihypertensive drugs, and calcium channel blockers. Insulin, levodopa, and tricyclic antidepressants can also cause vasodilation and orthostatic hypotension in predisposed patients. Poon and Braun,6 in a retrospective study in elderly veterans, identified hydrochlorothiazide, lisinopril (Prinivil, Zestril), trazodone (Desyrel), furosemide (Lasix), and terazosin (Hytrin) as the most common culprits.
ORTHOSTATIC HYPOTENSION IS COMMON IN THE ELDERLY
The prevalence of orthostatic hypotension is high in the elderly and depends on the characteristics of the population studied, such as age, use of medications, and comorbidities known to be associated with this problem. Orthostatic hypotension is more common in institutionalized elderly people (up to 68%)7 than in those living in the community (6%).8 The high prevalence among institutionalized patients likely reflects multiple disease processes, including neurologic and cardiac conditions, as well as medications associated with orthostatic hypotension.
CLINICAL MANIFESTATIONS ARE DUE TO HYPOPERFUSION, OVERCOMPENSATION
Symptoms are related to cerebral hypoperfusion, with resulting lack of cerebral oxygenation (causing lightheadedness, dizziness, weakness, difficulty thinking, headache, syncope, or feeling faint) and a compensatory autonomic overreaction (causing palpitations, tremulousness, nausea, coldness of extremities, chest pain, and syncope).
Lightheadedness is a common symptom, but subtler issues such as difficulty thinking, weakness, and neck discomfort are also common in the elderly. Recurrent or unexplained falls in older adults may be a manifestation of syncope due to orthostatic hypotension.
PROGNOSIS DEPENDS ON CAUSE
Orthostatic hypotension is a syndrome, and its prognosis depends on its specific cause, its severity, and the distribution of its autonomic and nonautonomic involvement. In patients who have extrapyramidal and cerebellar disorders (eg, Parkinson disease, multiple system atrophy), the earlier and the more severe the involvement of the autonomic nervous system, the poorer the prognosis.9,10
In hypertensive patients with diabetes mellitus, the risk of death is higher if they have orthostatic hypotension.11 Diastolic orthostatic hypotension is associated with a higher risk of vascular death in older persons.12
MANAGEMENT: FROM A TO F
The goal of management of orthostatic hypotension is to raise the patient’s standing blood pressure without also raising his or her supine blood pressure, and specifically to reduce orthostatic symptoms, increase the time the patient can stand, and improve his or her ability to perform daily activities. No specific treatment is currently available that achieves all these goals, and drugs alone are never completely adequate.
Because the mainstays of treatment are volume expansion and vasoconstriction, it is difficult to improve the symptoms of orthostatic hypotension without inducing some degree of supine hypertension. Strategies to minimize nocturnal hypertension and to treat orthostatic hypotension in special circumstances are summarized in Table 1.
If hypovolemia is playing a major role, and the patient cannot ingest enough salt or plasma volume fails to increase despite salt supplementation, fludrocortisone (Florinef) should be considered. Untreated hypovolemia will decrease the efficacy of vasoconstrictor drugs.
Pyridostigmine (Mestinon) has a putative vasoconstrictor effect only during standing, but because its effect is modest it should be used in mild orthostatic hypotension that does not improve with nonpharmacologic measures and in moderate cases. Its effect can be enhanced with additional low doses of midodrine (ProAmatine). Midodrine with or without fludrocortisone should be used in severe orthostatic hypotension.
A: Abdominal compression
In conditions in which there is adrenergic denervation of vascular beds, there is an increase in vascular capacitance and peripheral venous pooling. Compression of capacitance beds (ie, the legs and abdomen) improves orthostatic symptoms.14 The improvement is due to a reduction of venous capacitance and an increase in total peripheral resistance.14
On standing, healthy adults experience an orthostatic shift of approximately 500 mL of blood to the lower extremities15 that, when added to an increased vascular capacitance in those with orthostatic hypotension, results in a relative state of hypovolemia.
Compression of the legs alone is not as beneficial as compression of the abdomen because the venous capacitance of the calves and thighs is relatively small compared with that of the splanchnic mesenteric bed, which accounts for 20% to 30% of total blood volume.16 Moreover, compression garments and stockings that are strong enough to produce a measurable effect on orthostatic hypotension are cumbersome to put on and uncomfortable to wear. Because some patients gain significant benefit from abdominal compression alone, this should be considered the first step in reducing venous capacitance.
In a laboratory experiment, Smit et al17 found that an elastic abdominal binder that exerted 15 to 20 mm Hg of pressure on the abdomen raised the standing blood pressure by about 11/6 mmHg, which was comparable to the effect of a gravity suit (such as those worn by fighter pilots to prevent syncope during violent aircraft maneuvers) inflated to 20 mm Hg—an increase of about 17/8 mm Hg. Higher gravity-suit pressures had a greater effect.
In practical terms, the binder should be tight enough to exert gentle pressure. It should be put on before rising from bed in the morning and taken off when lying supine, to avoid supine hypertension. Advantages are that a binder’s effects are immediate, its benefits can be easily assessed, and it can be used on an as-needed basis by patients who need it only during periods of prolonged orthostatic stress. Binders are also easy to fit and are available in most sporting good stores and on the Web (try searching for “abdominal binder”).
When abdominal compression alone is not enough, the addition of compression of the lower extremities can result in further benefits. This can be achieved by using compression garments that ideally extend to the waist or, at the least, to the proximal thigh.
B: Boluses of water
Rapidly drinking two 8-oz (500-mL) glasses of cold water helps expand plasma volume. It also, within a few minutes, elicits a significant pressor effect that is in part norepinephrine-mediated,18,19 increasing the standing systolic blood pressure by more than 20 mm Hg for about 2 hours and improving symptoms and orthostatic endurance.18,20 This easy technique can be used when prolonged standing is expected (eg, shopping).
B (continued): Bed up
The head of the bed of a patient with orthostatic hypotension should be elevated by 10 to 20 degrees or 4 inches (10 cm) to decrease nocturnal hypertension and nocturnal diuresis.21 During the day, adequate orthostatic stress, ie, upright activity, should be maintained. If patients are repeatedly tilted up, their orthostatic hypotension is gradually attenuated, presumably by increasing venomotor tone.22
C: Countermaneuvers
Physical countermaneuvers involve isometrically contracting the muscles below the waist for about 30 seconds at a time, which reduces venous capacitance, increases total peripheral resistance, and augments venous return to the heart.23,24 These countermeasures can help maintain blood pressure during daily activities and should be considered at the first symptoms of orthostatic intolerance and in situations of orthostatic stress (eg, standing for prolonged periods).
Specific techniques include23:
- Toe-raising
- Leg-crossing and contraction
- Thigh muscle co-contraction
- Bending at the waist
- Slow marching in place
- Leg elevation.
D: Drugs
Midodrine, a vasopressor, is effective and safe when used for treating neurogenic orthostatic hypotension.25 It has been shown to increase standing systolic blood pressure, reduce orthostatic lightheadedness, and increase standing and walking time.
A common starting dose is 5 mg three times a day; most patients respond best to 10 mg three times a day. As its duration of action is short (2 to 4 hours),25–27 it should be taken before arising in the morning, before lunch, and in the midafternoon. To avoid nocturnal supine hypertension, doses should not be taken after the midafternoon, and a dose should be omitted if the supine or sitting blood pressure is greater than 180/100 mm Hg.
Midodrine’s main side effects are supine hypertension, scalp paresthesias, and pilomotor reactions (goosebumps). Vasoconstrictors such as midodrine are ineffective when plasma volume is reduced.
Fludrocortisone is a synthetic mineralocorticoid that has a pressor effect as a result of its ability to expand plasma volume and increase vascular alpha-adrenoceptor sensitivity.28–30 This medication is helpful when plasma volume fails to adequately increase with salt supplementation31 and for patients who cannot ingest enough salt or do not respond adequately to midodrine.
The usual dose is 0.1 to 0.2 mg/day, but it may be increased to 0.4 to 0.6 mg/day in patients with refractory orthostatic hypotension.
If the patient gains 3 to 5 pounds (1.2–2.3 kg) and develops mild dependent edema, you can infer that the plasma volume has expanded adequately. However, in view of these effects, fludrocortisone is contraindicated in congestive heart failure and chronic renal failure. The potential risks are severe hypokalemia and excessive supine hypertension. Frequent monitoring of serum potassium, a diet high in potassium, and regular checks of supine blood pressure are advised, especially at higher doses, when added to midodrine, or in elderly patients who tend to poorly tolerate the medication.28,29,32
Pyridostigmine is a cholinesterase inhibitor that improves ganglionic neurotransmission in the sympathetic baroreflex pathway. Because this pathway is activated primarily during standing, this drug improves orthostatic hypotension and total peripheral resistance without aggravating supine hypertension. Because the pressor effect is modest, it is most adequate for patients with mild to moderate orthostatic hypotension.33,34
Dosing is started at 30 mg two to three times a day and is gradually increased to 60 mg three times a day. The drug’s effectiveness can be enhanced by combining each dose of pyridostigmine with 5 mg of midodrine without occurrence of supine hypertension.34 Mestinon Timespan, a 180-mg slow-release pyridostigmine tablet, can be taken once a day and may be a convenient alternative.
The main side effects are cholinergic (abdominal colic, diarrhea).
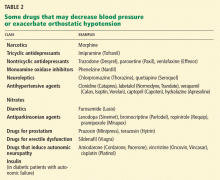
Review the patient’s medications. If he or she is taking any drug that may cause orthostatic hypotension, consider discontinuing it, substituting another drug, or changing the dosage (Table 2). In the elderly, antiparkinsonian, nitrate, antidepressant, diuretic, prostate, and antihypertensive medications35 may be particularly suspect.
E: Education
Education is probably the single most important factor in the proper control of orthostatic hypotension. A number of issues should be considered.
- Patients should be taught, in simple terms, the mechanisms that maintain postural normotension and how to recognize the onset of orthostatic symptoms.
- They must realize that there is no specific treatment of the underlying cause and that drug treatment alone is not adequate.
- They should be taught nonpharmacologic approaches and be aware that other drugs they start may worsen symptoms.
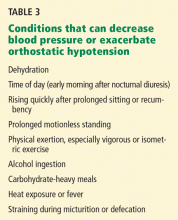
It is also important that the patient learn the conditions (and their mechanisms) that can lower blood pressure (Table 3). Such conditions include prolonged or motionless standing, alcohol ingestion (causing vasodilation), carbohydrate-heavy meals (causing postprandial orthostatic hypotension related to an increase in the splanchnic-mesenteric venous capacitance), early morning orthostatic hypotension related to nocturnal diuresis and arising from bed, physical activity sufficient to cause muscle vasodilation, heat exposure (eg, hot weather or a hot bath or shower) producing skin vessel vasodilation, sudden postural changes, and prolonged recumbency. Once these stressors are explained, patients have no difficulty recognizing them.
The patient should also be instructed in how to manage situations of increased orthostatic stress and periods of orthostatic decompensation, to minimize nocturnal hypertension, and to modify their activities of daily living. Keeping a log of supine and upright blood pressures (taken with an automated sphygmomanometer) during situations of orthostatic stress can help establish whether worsening symptoms are related to orthostatic hypotension or to another mechanism. Once patients discover that they can actively deal with these situations, they develop a great sense of empowerment.
E (continued): Exercise
Mild physical exercise improves orthostatic tolerance by reducing venous pooling and increasing plasma volume.36 Deconditioning from lack of exercise exacerbates orthostatic hypotension.37 Because upright exercise may increase the orthostatic drop in blood pressure, training in a supine or sitting position (eg, swimming, recumbent biking) is advisable. Isotonic exercise (eg, light weight-lifting) is recommended because the incorrect straining and breath-holding during isometric exercise (eg, holding weights in the same position) may decrease venous return.
F: Fluid and salt (volume expansion)
Maintaining an adequate plasma volume is crucial. Patients should drink five to eight 8-ounce glasses (1.25 to 2.5 L) of water or other fluid per day. Many elderly people do not take in this much. The patient should have at least 1 glass or cup of fluid with meals and at least twice at other times of each day to obtain 1 L/day.
Salt intake should be between 150 and 250 mmol of sodium (10 to 20 g of salt) per day. Sodium helps with retention of ingested fluids and should be maximized if tolerated. However, caution should be exercised in patients who have severe refractory supine hypertension, uncontrolled hypertension, or comorbidities characterized by insterstitial edema (eg, heart failure, liver failure). Some patients are very sensitive to sodium supplementation and can fine-tune their orthostatic control with salt alone. If salting food is not desired, prepared soups, pretzels, potato chips, and 0.5- or 1.0-g salt tablets can be an option.
Patients need to maintain a high-potassium diet, as the high sodium intake combined with fludrocortisone promotes potassium loss. Fruits (especially bananas) and vegetables have high potassium content.
The combination of fludrocortisone and a high-salt diet can also cause sustained supine hypertension, which can be minimized by the interventions noted in Table 2.
Appropriate salt supplementation and fluid intake leading to an adequate volume expansion can be verified by checking the 24-hour urinary sodium content: patients who excrete less than 170 mmol can be treated with 1 to 2 g of supplemental sodium three times a day.38
- Sjostrand T. The regulation of the blood distribution in man. Acta Physiol Scand 1952; 26:312–327.
- Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med 1977; 296:293–297.
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46:1470.
- Gehrking JA, Hines SM, Benrud-Larson LM, Opher-Gehrking TL, Low PA. What is the minimum duration of head-up tilt necessary to detect orthostatic hypotension? Clin Auton Res 2005; 15:71–75.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 2005; 30:173–178.
- Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med 2002; 162:2369–2374.
- Mader SL, Josephson KR, Rubenstein LZ. Low prevalence of postural hypotension among community-dwelling elderly. JAMA 1987; 258:1511–1514.
- Sandroni P, Ahlskog JE, Fealey RD, Low PA. Autonomic involvement in extrapyramidal and cerebellar disorders. Clin Auton Res 1991; 1:147–155.
- Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med 1994; 33:321–325.
- Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation 1987; 75:340–346.
- Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med 1999; 159:273–280.
- Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med 1993; 329:611–615.
- Denq JC, Opfer-Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res 1997; 7:321–326.
- Sjostrand T. Volume and distribution of blood and their significance in regulating the circulation. Physiol Rev 1953; 33:202–228.
- Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol 1972; 32:213–220.
- Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004; 14:167–175.
- Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation 2000; 101:504–509.
- Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med 2002; 112:355–360.
- Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water [letter]. Lancet 1999; 353:723.
- MacLean AR, Allen EV. Orthostatic hypotension and orthostatic tachycardia: treatment with the “head-up” bed. JAMA 1940; 115:2162–2167.
- Ector H, Reybrouck T, Heidbüchel H, Gewillig M, Van de Werf F. Tilt training: a new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pacing Clin Electrophysiol 1998; 21:193–196.
- Bouvette CM, McPhee BR, Opfer-Gehrking TL, Low PA. Role of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentation. Mayo Clin Proc 1996; 71:847–853.
- Ten Harkel AD, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci (Lond) 1994; 87:553–558.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med 1993; 95:38–48.
- Fouad-Tarazi FM, Okabe M, Goren H. Alpha sympathomimetic treatment of autonomic insufficiency with orthostatic hypotension. Am J Med 1995; 99:604–610.
- Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets 2007; 7:63–70.
- Axelrod FB, Goldberg JD, Rolnitzky L, et al. Fludrocortisone in patients with familial dysautonomia—assessing effect on clinical parameters and gene expression. Clin Auton Res 2005; 15:284–291.
- Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. N Engl J Med 1979; 301:68–73.
- van Lieshout JJ, Ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res 2000; 10:35–42.
- Hussain RM, McIntosh SJ, Lawson J, Kenny RA. Fludrocortisone in the treatment of hypotensive disorders in the elderly. Heart 1996; 76:507–509.
- Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry 2003; 74:1294–1298.
- Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006; 63:513–518.
- Lipsitz LA, Pluchino FC, Wei JY, Rowe JW. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis 1986; 39:619–630.
- Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol 1999; 84:121–130.
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol 2001; 85:420–426.
- El-Sayed H, Hainsworth R. Salt supplementation increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart 1996; 75:134–140.
Orthostatic hypotension is a chronic, debilitating illness associated with common neurologic conditions (eg, diabetic neuropathy, Parkinson disease). It is common in the elderly, especially in those who are institutionalized and are using multiple medications.
Treatment can be challenging, especially if the problem is neurogenic. This condition has no cure, symptoms vary in different circumstances, treatment is nonspecific, and aggressive treatment can lead to marked supine hypertension.
This review focuses on the prevention and treatment of neurogenic causes of orthostatic hypotension. We emphasize a simple but effective patient-oriented approach to management, using a combination of nonpharmacologic strategies and drugs clinically proven to be efficacious. The recommendations and their rationale are organized in a practical and easy-to-remember format for both physicians and patients.
WHAT HAPPENS WHEN WE STAND UP?
When we stand up, the blood goes down from the chest to the distensible venous capacitance system below the diaphragm. This fluid shift produces a decrease in venous return, ventricular filling, cardiac output, and blood pressure.1
This gravity-induced drop in blood pressure, detected by arterial baroreceptors in the aortic arch and carotid sinus, triggers a compensatory reflex tachycardia and vasoconstriction that restores normotension in the upright position. This compensatory mechanism is termed a baroreflex; it is mediated by afferent and efferent autonomic peripheral nerves and is integrated in autonomic centers in the brainstem.2
Orthostatic hypotension is the result of baroreflex failure (autonomic failure), end-organ dysfunction, or volume depletion. Injury to any limb of the baroreflex causes neurogenic orthostatic hypotension, although with afferent lesions alone, the hypotension tends to be modest and accompanied by wide fluctuations in blood pressure, including severe hypertension. Drugs can produce orthostatic hypotension by interfering with the autonomic pathways or their target end-organs or by affecting intravascular volume. Brain hypoperfusion, resulting from orthostatic hypotension from any cause, can lead to symptoms of orthostatic intolerance (eg, lightheadedness) and falls, and if the hypotension is severe, to syncope.
A DECREASE OF 20 MM HG SYSTOLIC OR 10 MM HG DIASTOLIC
The consensus definition of orthostatic hypotension is a reduction of systolic blood pressure of at least 20 mm Hg or a reduction of diastolic blood pressure of at least 10 mm Hg within 3 minutes of erect standing.3 A transient drop that occurs with abrupt standing and resolves rapidly suggests a benign condition, such as dehydration, rather than autonomic failure.
In the laboratory, patients are placed on a tilt table in the head-up position at an angle of at least 60 degrees to detect orthostatic changes in blood pressure. In the office, 1 minute of standing probably detects nearly all cases of orthostatic hypotension; however, standing beyond 2 minutes helps establish the severity (a further drop in blood pressure).4 Orthostatic hypotension developing after 3 minutes of standing is uncommon and may represent a reflex presyncope (eg, vasovagal) or a mild or early form of sympathetic adrenergic dysfunction.4, 5
NEUROGENIC AND NONNEUROGENIC CAUSES
Orthostatic hypotension may result from neurogenic and nonneurogenic causes.
Neurogenic orthostatic hypotension can be due to neuropathy (eg, diabetic or autoimmune neuropathies) or to central lesions (eg, Parkinson disease or multiple system atrophy). Its presence, severity, and temporal course can be important clues in diagnosing Parkinson disease and differentiating it from other parkinsonian syndromes with a more ominous prognosis, such as multiple system atrophy and Lewy body dementia.
Nonneurogenic causes include cardiac impairment (eg, from myocardial infarction or aortic stenosis), reduced intravascular volume (eg, from dehydration, adrenal insufficiency), and vasodilation (eg, from fever, systemic mastocytosis).
Common drugs that cause orthostatic hypotension are diuretics, alpha-adrenoceptor blockers for prostatic hypertrophy, antihypertensive drugs, and calcium channel blockers. Insulin, levodopa, and tricyclic antidepressants can also cause vasodilation and orthostatic hypotension in predisposed patients. Poon and Braun,6 in a retrospective study in elderly veterans, identified hydrochlorothiazide, lisinopril (Prinivil, Zestril), trazodone (Desyrel), furosemide (Lasix), and terazosin (Hytrin) as the most common culprits.
ORTHOSTATIC HYPOTENSION IS COMMON IN THE ELDERLY
The prevalence of orthostatic hypotension is high in the elderly and depends on the characteristics of the population studied, such as age, use of medications, and comorbidities known to be associated with this problem. Orthostatic hypotension is more common in institutionalized elderly people (up to 68%)7 than in those living in the community (6%).8 The high prevalence among institutionalized patients likely reflects multiple disease processes, including neurologic and cardiac conditions, as well as medications associated with orthostatic hypotension.
CLINICAL MANIFESTATIONS ARE DUE TO HYPOPERFUSION, OVERCOMPENSATION
Symptoms are related to cerebral hypoperfusion, with resulting lack of cerebral oxygenation (causing lightheadedness, dizziness, weakness, difficulty thinking, headache, syncope, or feeling faint) and a compensatory autonomic overreaction (causing palpitations, tremulousness, nausea, coldness of extremities, chest pain, and syncope).
Lightheadedness is a common symptom, but subtler issues such as difficulty thinking, weakness, and neck discomfort are also common in the elderly. Recurrent or unexplained falls in older adults may be a manifestation of syncope due to orthostatic hypotension.
PROGNOSIS DEPENDS ON CAUSE
Orthostatic hypotension is a syndrome, and its prognosis depends on its specific cause, its severity, and the distribution of its autonomic and nonautonomic involvement. In patients who have extrapyramidal and cerebellar disorders (eg, Parkinson disease, multiple system atrophy), the earlier and the more severe the involvement of the autonomic nervous system, the poorer the prognosis.9,10
In hypertensive patients with diabetes mellitus, the risk of death is higher if they have orthostatic hypotension.11 Diastolic orthostatic hypotension is associated with a higher risk of vascular death in older persons.12
MANAGEMENT: FROM A TO F
The goal of management of orthostatic hypotension is to raise the patient’s standing blood pressure without also raising his or her supine blood pressure, and specifically to reduce orthostatic symptoms, increase the time the patient can stand, and improve his or her ability to perform daily activities. No specific treatment is currently available that achieves all these goals, and drugs alone are never completely adequate.
Because the mainstays of treatment are volume expansion and vasoconstriction, it is difficult to improve the symptoms of orthostatic hypotension without inducing some degree of supine hypertension. Strategies to minimize nocturnal hypertension and to treat orthostatic hypotension in special circumstances are summarized in Table 1.
If hypovolemia is playing a major role, and the patient cannot ingest enough salt or plasma volume fails to increase despite salt supplementation, fludrocortisone (Florinef) should be considered. Untreated hypovolemia will decrease the efficacy of vasoconstrictor drugs.
Pyridostigmine (Mestinon) has a putative vasoconstrictor effect only during standing, but because its effect is modest it should be used in mild orthostatic hypotension that does not improve with nonpharmacologic measures and in moderate cases. Its effect can be enhanced with additional low doses of midodrine (ProAmatine). Midodrine with or without fludrocortisone should be used in severe orthostatic hypotension.
A: Abdominal compression
In conditions in which there is adrenergic denervation of vascular beds, there is an increase in vascular capacitance and peripheral venous pooling. Compression of capacitance beds (ie, the legs and abdomen) improves orthostatic symptoms.14 The improvement is due to a reduction of venous capacitance and an increase in total peripheral resistance.14
On standing, healthy adults experience an orthostatic shift of approximately 500 mL of blood to the lower extremities15 that, when added to an increased vascular capacitance in those with orthostatic hypotension, results in a relative state of hypovolemia.
Compression of the legs alone is not as beneficial as compression of the abdomen because the venous capacitance of the calves and thighs is relatively small compared with that of the splanchnic mesenteric bed, which accounts for 20% to 30% of total blood volume.16 Moreover, compression garments and stockings that are strong enough to produce a measurable effect on orthostatic hypotension are cumbersome to put on and uncomfortable to wear. Because some patients gain significant benefit from abdominal compression alone, this should be considered the first step in reducing venous capacitance.
In a laboratory experiment, Smit et al17 found that an elastic abdominal binder that exerted 15 to 20 mm Hg of pressure on the abdomen raised the standing blood pressure by about 11/6 mmHg, which was comparable to the effect of a gravity suit (such as those worn by fighter pilots to prevent syncope during violent aircraft maneuvers) inflated to 20 mm Hg—an increase of about 17/8 mm Hg. Higher gravity-suit pressures had a greater effect.
In practical terms, the binder should be tight enough to exert gentle pressure. It should be put on before rising from bed in the morning and taken off when lying supine, to avoid supine hypertension. Advantages are that a binder’s effects are immediate, its benefits can be easily assessed, and it can be used on an as-needed basis by patients who need it only during periods of prolonged orthostatic stress. Binders are also easy to fit and are available in most sporting good stores and on the Web (try searching for “abdominal binder”).
When abdominal compression alone is not enough, the addition of compression of the lower extremities can result in further benefits. This can be achieved by using compression garments that ideally extend to the waist or, at the least, to the proximal thigh.
B: Boluses of water
Rapidly drinking two 8-oz (500-mL) glasses of cold water helps expand plasma volume. It also, within a few minutes, elicits a significant pressor effect that is in part norepinephrine-mediated,18,19 increasing the standing systolic blood pressure by more than 20 mm Hg for about 2 hours and improving symptoms and orthostatic endurance.18,20 This easy technique can be used when prolonged standing is expected (eg, shopping).
B (continued): Bed up
The head of the bed of a patient with orthostatic hypotension should be elevated by 10 to 20 degrees or 4 inches (10 cm) to decrease nocturnal hypertension and nocturnal diuresis.21 During the day, adequate orthostatic stress, ie, upright activity, should be maintained. If patients are repeatedly tilted up, their orthostatic hypotension is gradually attenuated, presumably by increasing venomotor tone.22
C: Countermaneuvers
Physical countermaneuvers involve isometrically contracting the muscles below the waist for about 30 seconds at a time, which reduces venous capacitance, increases total peripheral resistance, and augments venous return to the heart.23,24 These countermeasures can help maintain blood pressure during daily activities and should be considered at the first symptoms of orthostatic intolerance and in situations of orthostatic stress (eg, standing for prolonged periods).
Specific techniques include23:
- Toe-raising
- Leg-crossing and contraction
- Thigh muscle co-contraction
- Bending at the waist
- Slow marching in place
- Leg elevation.
D: Drugs
Midodrine, a vasopressor, is effective and safe when used for treating neurogenic orthostatic hypotension.25 It has been shown to increase standing systolic blood pressure, reduce orthostatic lightheadedness, and increase standing and walking time.
A common starting dose is 5 mg three times a day; most patients respond best to 10 mg three times a day. As its duration of action is short (2 to 4 hours),25–27 it should be taken before arising in the morning, before lunch, and in the midafternoon. To avoid nocturnal supine hypertension, doses should not be taken after the midafternoon, and a dose should be omitted if the supine or sitting blood pressure is greater than 180/100 mm Hg.
Midodrine’s main side effects are supine hypertension, scalp paresthesias, and pilomotor reactions (goosebumps). Vasoconstrictors such as midodrine are ineffective when plasma volume is reduced.
Fludrocortisone is a synthetic mineralocorticoid that has a pressor effect as a result of its ability to expand plasma volume and increase vascular alpha-adrenoceptor sensitivity.28–30 This medication is helpful when plasma volume fails to adequately increase with salt supplementation31 and for patients who cannot ingest enough salt or do not respond adequately to midodrine.
The usual dose is 0.1 to 0.2 mg/day, but it may be increased to 0.4 to 0.6 mg/day in patients with refractory orthostatic hypotension.
If the patient gains 3 to 5 pounds (1.2–2.3 kg) and develops mild dependent edema, you can infer that the plasma volume has expanded adequately. However, in view of these effects, fludrocortisone is contraindicated in congestive heart failure and chronic renal failure. The potential risks are severe hypokalemia and excessive supine hypertension. Frequent monitoring of serum potassium, a diet high in potassium, and regular checks of supine blood pressure are advised, especially at higher doses, when added to midodrine, or in elderly patients who tend to poorly tolerate the medication.28,29,32
Pyridostigmine is a cholinesterase inhibitor that improves ganglionic neurotransmission in the sympathetic baroreflex pathway. Because this pathway is activated primarily during standing, this drug improves orthostatic hypotension and total peripheral resistance without aggravating supine hypertension. Because the pressor effect is modest, it is most adequate for patients with mild to moderate orthostatic hypotension.33,34
Dosing is started at 30 mg two to three times a day and is gradually increased to 60 mg three times a day. The drug’s effectiveness can be enhanced by combining each dose of pyridostigmine with 5 mg of midodrine without occurrence of supine hypertension.34 Mestinon Timespan, a 180-mg slow-release pyridostigmine tablet, can be taken once a day and may be a convenient alternative.
The main side effects are cholinergic (abdominal colic, diarrhea).
Review the patient’s medications. If he or she is taking any drug that may cause orthostatic hypotension, consider discontinuing it, substituting another drug, or changing the dosage (Table 2). In the elderly, antiparkinsonian, nitrate, antidepressant, diuretic, prostate, and antihypertensive medications35 may be particularly suspect.
E: Education
Education is probably the single most important factor in the proper control of orthostatic hypotension. A number of issues should be considered.
- Patients should be taught, in simple terms, the mechanisms that maintain postural normotension and how to recognize the onset of orthostatic symptoms.
- They must realize that there is no specific treatment of the underlying cause and that drug treatment alone is not adequate.
- They should be taught nonpharmacologic approaches and be aware that other drugs they start may worsen symptoms.
It is also important that the patient learn the conditions (and their mechanisms) that can lower blood pressure (Table 3). Such conditions include prolonged or motionless standing, alcohol ingestion (causing vasodilation), carbohydrate-heavy meals (causing postprandial orthostatic hypotension related to an increase in the splanchnic-mesenteric venous capacitance), early morning orthostatic hypotension related to nocturnal diuresis and arising from bed, physical activity sufficient to cause muscle vasodilation, heat exposure (eg, hot weather or a hot bath or shower) producing skin vessel vasodilation, sudden postural changes, and prolonged recumbency. Once these stressors are explained, patients have no difficulty recognizing them.
The patient should also be instructed in how to manage situations of increased orthostatic stress and periods of orthostatic decompensation, to minimize nocturnal hypertension, and to modify their activities of daily living. Keeping a log of supine and upright blood pressures (taken with an automated sphygmomanometer) during situations of orthostatic stress can help establish whether worsening symptoms are related to orthostatic hypotension or to another mechanism. Once patients discover that they can actively deal with these situations, they develop a great sense of empowerment.
E (continued): Exercise
Mild physical exercise improves orthostatic tolerance by reducing venous pooling and increasing plasma volume.36 Deconditioning from lack of exercise exacerbates orthostatic hypotension.37 Because upright exercise may increase the orthostatic drop in blood pressure, training in a supine or sitting position (eg, swimming, recumbent biking) is advisable. Isotonic exercise (eg, light weight-lifting) is recommended because the incorrect straining and breath-holding during isometric exercise (eg, holding weights in the same position) may decrease venous return.
F: Fluid and salt (volume expansion)
Maintaining an adequate plasma volume is crucial. Patients should drink five to eight 8-ounce glasses (1.25 to 2.5 L) of water or other fluid per day. Many elderly people do not take in this much. The patient should have at least 1 glass or cup of fluid with meals and at least twice at other times of each day to obtain 1 L/day.
Salt intake should be between 150 and 250 mmol of sodium (10 to 20 g of salt) per day. Sodium helps with retention of ingested fluids and should be maximized if tolerated. However, caution should be exercised in patients who have severe refractory supine hypertension, uncontrolled hypertension, or comorbidities characterized by insterstitial edema (eg, heart failure, liver failure). Some patients are very sensitive to sodium supplementation and can fine-tune their orthostatic control with salt alone. If salting food is not desired, prepared soups, pretzels, potato chips, and 0.5- or 1.0-g salt tablets can be an option.
Patients need to maintain a high-potassium diet, as the high sodium intake combined with fludrocortisone promotes potassium loss. Fruits (especially bananas) and vegetables have high potassium content.
The combination of fludrocortisone and a high-salt diet can also cause sustained supine hypertension, which can be minimized by the interventions noted in Table 2.
Appropriate salt supplementation and fluid intake leading to an adequate volume expansion can be verified by checking the 24-hour urinary sodium content: patients who excrete less than 170 mmol can be treated with 1 to 2 g of supplemental sodium three times a day.38
Orthostatic hypotension is a chronic, debilitating illness associated with common neurologic conditions (eg, diabetic neuropathy, Parkinson disease). It is common in the elderly, especially in those who are institutionalized and are using multiple medications.
Treatment can be challenging, especially if the problem is neurogenic. This condition has no cure, symptoms vary in different circumstances, treatment is nonspecific, and aggressive treatment can lead to marked supine hypertension.
This review focuses on the prevention and treatment of neurogenic causes of orthostatic hypotension. We emphasize a simple but effective patient-oriented approach to management, using a combination of nonpharmacologic strategies and drugs clinically proven to be efficacious. The recommendations and their rationale are organized in a practical and easy-to-remember format for both physicians and patients.
WHAT HAPPENS WHEN WE STAND UP?
When we stand up, the blood goes down from the chest to the distensible venous capacitance system below the diaphragm. This fluid shift produces a decrease in venous return, ventricular filling, cardiac output, and blood pressure.1
This gravity-induced drop in blood pressure, detected by arterial baroreceptors in the aortic arch and carotid sinus, triggers a compensatory reflex tachycardia and vasoconstriction that restores normotension in the upright position. This compensatory mechanism is termed a baroreflex; it is mediated by afferent and efferent autonomic peripheral nerves and is integrated in autonomic centers in the brainstem.2
Orthostatic hypotension is the result of baroreflex failure (autonomic failure), end-organ dysfunction, or volume depletion. Injury to any limb of the baroreflex causes neurogenic orthostatic hypotension, although with afferent lesions alone, the hypotension tends to be modest and accompanied by wide fluctuations in blood pressure, including severe hypertension. Drugs can produce orthostatic hypotension by interfering with the autonomic pathways or their target end-organs or by affecting intravascular volume. Brain hypoperfusion, resulting from orthostatic hypotension from any cause, can lead to symptoms of orthostatic intolerance (eg, lightheadedness) and falls, and if the hypotension is severe, to syncope.
A DECREASE OF 20 MM HG SYSTOLIC OR 10 MM HG DIASTOLIC
The consensus definition of orthostatic hypotension is a reduction of systolic blood pressure of at least 20 mm Hg or a reduction of diastolic blood pressure of at least 10 mm Hg within 3 minutes of erect standing.3 A transient drop that occurs with abrupt standing and resolves rapidly suggests a benign condition, such as dehydration, rather than autonomic failure.
In the laboratory, patients are placed on a tilt table in the head-up position at an angle of at least 60 degrees to detect orthostatic changes in blood pressure. In the office, 1 minute of standing probably detects nearly all cases of orthostatic hypotension; however, standing beyond 2 minutes helps establish the severity (a further drop in blood pressure).4 Orthostatic hypotension developing after 3 minutes of standing is uncommon and may represent a reflex presyncope (eg, vasovagal) or a mild or early form of sympathetic adrenergic dysfunction.4, 5
NEUROGENIC AND NONNEUROGENIC CAUSES
Orthostatic hypotension may result from neurogenic and nonneurogenic causes.
Neurogenic orthostatic hypotension can be due to neuropathy (eg, diabetic or autoimmune neuropathies) or to central lesions (eg, Parkinson disease or multiple system atrophy). Its presence, severity, and temporal course can be important clues in diagnosing Parkinson disease and differentiating it from other parkinsonian syndromes with a more ominous prognosis, such as multiple system atrophy and Lewy body dementia.
Nonneurogenic causes include cardiac impairment (eg, from myocardial infarction or aortic stenosis), reduced intravascular volume (eg, from dehydration, adrenal insufficiency), and vasodilation (eg, from fever, systemic mastocytosis).
Common drugs that cause orthostatic hypotension are diuretics, alpha-adrenoceptor blockers for prostatic hypertrophy, antihypertensive drugs, and calcium channel blockers. Insulin, levodopa, and tricyclic antidepressants can also cause vasodilation and orthostatic hypotension in predisposed patients. Poon and Braun,6 in a retrospective study in elderly veterans, identified hydrochlorothiazide, lisinopril (Prinivil, Zestril), trazodone (Desyrel), furosemide (Lasix), and terazosin (Hytrin) as the most common culprits.
ORTHOSTATIC HYPOTENSION IS COMMON IN THE ELDERLY
The prevalence of orthostatic hypotension is high in the elderly and depends on the characteristics of the population studied, such as age, use of medications, and comorbidities known to be associated with this problem. Orthostatic hypotension is more common in institutionalized elderly people (up to 68%)7 than in those living in the community (6%).8 The high prevalence among institutionalized patients likely reflects multiple disease processes, including neurologic and cardiac conditions, as well as medications associated with orthostatic hypotension.
CLINICAL MANIFESTATIONS ARE DUE TO HYPOPERFUSION, OVERCOMPENSATION
Symptoms are related to cerebral hypoperfusion, with resulting lack of cerebral oxygenation (causing lightheadedness, dizziness, weakness, difficulty thinking, headache, syncope, or feeling faint) and a compensatory autonomic overreaction (causing palpitations, tremulousness, nausea, coldness of extremities, chest pain, and syncope).
Lightheadedness is a common symptom, but subtler issues such as difficulty thinking, weakness, and neck discomfort are also common in the elderly. Recurrent or unexplained falls in older adults may be a manifestation of syncope due to orthostatic hypotension.
PROGNOSIS DEPENDS ON CAUSE
Orthostatic hypotension is a syndrome, and its prognosis depends on its specific cause, its severity, and the distribution of its autonomic and nonautonomic involvement. In patients who have extrapyramidal and cerebellar disorders (eg, Parkinson disease, multiple system atrophy), the earlier and the more severe the involvement of the autonomic nervous system, the poorer the prognosis.9,10
In hypertensive patients with diabetes mellitus, the risk of death is higher if they have orthostatic hypotension.11 Diastolic orthostatic hypotension is associated with a higher risk of vascular death in older persons.12
MANAGEMENT: FROM A TO F
The goal of management of orthostatic hypotension is to raise the patient’s standing blood pressure without also raising his or her supine blood pressure, and specifically to reduce orthostatic symptoms, increase the time the patient can stand, and improve his or her ability to perform daily activities. No specific treatment is currently available that achieves all these goals, and drugs alone are never completely adequate.
Because the mainstays of treatment are volume expansion and vasoconstriction, it is difficult to improve the symptoms of orthostatic hypotension without inducing some degree of supine hypertension. Strategies to minimize nocturnal hypertension and to treat orthostatic hypotension in special circumstances are summarized in Table 1.
If hypovolemia is playing a major role, and the patient cannot ingest enough salt or plasma volume fails to increase despite salt supplementation, fludrocortisone (Florinef) should be considered. Untreated hypovolemia will decrease the efficacy of vasoconstrictor drugs.
Pyridostigmine (Mestinon) has a putative vasoconstrictor effect only during standing, but because its effect is modest it should be used in mild orthostatic hypotension that does not improve with nonpharmacologic measures and in moderate cases. Its effect can be enhanced with additional low doses of midodrine (ProAmatine). Midodrine with or without fludrocortisone should be used in severe orthostatic hypotension.
A: Abdominal compression
In conditions in which there is adrenergic denervation of vascular beds, there is an increase in vascular capacitance and peripheral venous pooling. Compression of capacitance beds (ie, the legs and abdomen) improves orthostatic symptoms.14 The improvement is due to a reduction of venous capacitance and an increase in total peripheral resistance.14
On standing, healthy adults experience an orthostatic shift of approximately 500 mL of blood to the lower extremities15 that, when added to an increased vascular capacitance in those with orthostatic hypotension, results in a relative state of hypovolemia.
Compression of the legs alone is not as beneficial as compression of the abdomen because the venous capacitance of the calves and thighs is relatively small compared with that of the splanchnic mesenteric bed, which accounts for 20% to 30% of total blood volume.16 Moreover, compression garments and stockings that are strong enough to produce a measurable effect on orthostatic hypotension are cumbersome to put on and uncomfortable to wear. Because some patients gain significant benefit from abdominal compression alone, this should be considered the first step in reducing venous capacitance.
In a laboratory experiment, Smit et al17 found that an elastic abdominal binder that exerted 15 to 20 mm Hg of pressure on the abdomen raised the standing blood pressure by about 11/6 mmHg, which was comparable to the effect of a gravity suit (such as those worn by fighter pilots to prevent syncope during violent aircraft maneuvers) inflated to 20 mm Hg—an increase of about 17/8 mm Hg. Higher gravity-suit pressures had a greater effect.
In practical terms, the binder should be tight enough to exert gentle pressure. It should be put on before rising from bed in the morning and taken off when lying supine, to avoid supine hypertension. Advantages are that a binder’s effects are immediate, its benefits can be easily assessed, and it can be used on an as-needed basis by patients who need it only during periods of prolonged orthostatic stress. Binders are also easy to fit and are available in most sporting good stores and on the Web (try searching for “abdominal binder”).
When abdominal compression alone is not enough, the addition of compression of the lower extremities can result in further benefits. This can be achieved by using compression garments that ideally extend to the waist or, at the least, to the proximal thigh.
B: Boluses of water
Rapidly drinking two 8-oz (500-mL) glasses of cold water helps expand plasma volume. It also, within a few minutes, elicits a significant pressor effect that is in part norepinephrine-mediated,18,19 increasing the standing systolic blood pressure by more than 20 mm Hg for about 2 hours and improving symptoms and orthostatic endurance.18,20 This easy technique can be used when prolonged standing is expected (eg, shopping).
B (continued): Bed up
The head of the bed of a patient with orthostatic hypotension should be elevated by 10 to 20 degrees or 4 inches (10 cm) to decrease nocturnal hypertension and nocturnal diuresis.21 During the day, adequate orthostatic stress, ie, upright activity, should be maintained. If patients are repeatedly tilted up, their orthostatic hypotension is gradually attenuated, presumably by increasing venomotor tone.22
C: Countermaneuvers
Physical countermaneuvers involve isometrically contracting the muscles below the waist for about 30 seconds at a time, which reduces venous capacitance, increases total peripheral resistance, and augments venous return to the heart.23,24 These countermeasures can help maintain blood pressure during daily activities and should be considered at the first symptoms of orthostatic intolerance and in situations of orthostatic stress (eg, standing for prolonged periods).
Specific techniques include23:
- Toe-raising
- Leg-crossing and contraction
- Thigh muscle co-contraction
- Bending at the waist
- Slow marching in place
- Leg elevation.
D: Drugs
Midodrine, a vasopressor, is effective and safe when used for treating neurogenic orthostatic hypotension.25 It has been shown to increase standing systolic blood pressure, reduce orthostatic lightheadedness, and increase standing and walking time.
A common starting dose is 5 mg three times a day; most patients respond best to 10 mg three times a day. As its duration of action is short (2 to 4 hours),25–27 it should be taken before arising in the morning, before lunch, and in the midafternoon. To avoid nocturnal supine hypertension, doses should not be taken after the midafternoon, and a dose should be omitted if the supine or sitting blood pressure is greater than 180/100 mm Hg.
Midodrine’s main side effects are supine hypertension, scalp paresthesias, and pilomotor reactions (goosebumps). Vasoconstrictors such as midodrine are ineffective when plasma volume is reduced.
Fludrocortisone is a synthetic mineralocorticoid that has a pressor effect as a result of its ability to expand plasma volume and increase vascular alpha-adrenoceptor sensitivity.28–30 This medication is helpful when plasma volume fails to adequately increase with salt supplementation31 and for patients who cannot ingest enough salt or do not respond adequately to midodrine.
The usual dose is 0.1 to 0.2 mg/day, but it may be increased to 0.4 to 0.6 mg/day in patients with refractory orthostatic hypotension.
If the patient gains 3 to 5 pounds (1.2–2.3 kg) and develops mild dependent edema, you can infer that the plasma volume has expanded adequately. However, in view of these effects, fludrocortisone is contraindicated in congestive heart failure and chronic renal failure. The potential risks are severe hypokalemia and excessive supine hypertension. Frequent monitoring of serum potassium, a diet high in potassium, and regular checks of supine blood pressure are advised, especially at higher doses, when added to midodrine, or in elderly patients who tend to poorly tolerate the medication.28,29,32
Pyridostigmine is a cholinesterase inhibitor that improves ganglionic neurotransmission in the sympathetic baroreflex pathway. Because this pathway is activated primarily during standing, this drug improves orthostatic hypotension and total peripheral resistance without aggravating supine hypertension. Because the pressor effect is modest, it is most adequate for patients with mild to moderate orthostatic hypotension.33,34
Dosing is started at 30 mg two to three times a day and is gradually increased to 60 mg three times a day. The drug’s effectiveness can be enhanced by combining each dose of pyridostigmine with 5 mg of midodrine without occurrence of supine hypertension.34 Mestinon Timespan, a 180-mg slow-release pyridostigmine tablet, can be taken once a day and may be a convenient alternative.
The main side effects are cholinergic (abdominal colic, diarrhea).
Review the patient’s medications. If he or she is taking any drug that may cause orthostatic hypotension, consider discontinuing it, substituting another drug, or changing the dosage (Table 2). In the elderly, antiparkinsonian, nitrate, antidepressant, diuretic, prostate, and antihypertensive medications35 may be particularly suspect.
E: Education
Education is probably the single most important factor in the proper control of orthostatic hypotension. A number of issues should be considered.
- Patients should be taught, in simple terms, the mechanisms that maintain postural normotension and how to recognize the onset of orthostatic symptoms.
- They must realize that there is no specific treatment of the underlying cause and that drug treatment alone is not adequate.
- They should be taught nonpharmacologic approaches and be aware that other drugs they start may worsen symptoms.
It is also important that the patient learn the conditions (and their mechanisms) that can lower blood pressure (Table 3). Such conditions include prolonged or motionless standing, alcohol ingestion (causing vasodilation), carbohydrate-heavy meals (causing postprandial orthostatic hypotension related to an increase in the splanchnic-mesenteric venous capacitance), early morning orthostatic hypotension related to nocturnal diuresis and arising from bed, physical activity sufficient to cause muscle vasodilation, heat exposure (eg, hot weather or a hot bath or shower) producing skin vessel vasodilation, sudden postural changes, and prolonged recumbency. Once these stressors are explained, patients have no difficulty recognizing them.
The patient should also be instructed in how to manage situations of increased orthostatic stress and periods of orthostatic decompensation, to minimize nocturnal hypertension, and to modify their activities of daily living. Keeping a log of supine and upright blood pressures (taken with an automated sphygmomanometer) during situations of orthostatic stress can help establish whether worsening symptoms are related to orthostatic hypotension or to another mechanism. Once patients discover that they can actively deal with these situations, they develop a great sense of empowerment.
E (continued): Exercise
Mild physical exercise improves orthostatic tolerance by reducing venous pooling and increasing plasma volume.36 Deconditioning from lack of exercise exacerbates orthostatic hypotension.37 Because upright exercise may increase the orthostatic drop in blood pressure, training in a supine or sitting position (eg, swimming, recumbent biking) is advisable. Isotonic exercise (eg, light weight-lifting) is recommended because the incorrect straining and breath-holding during isometric exercise (eg, holding weights in the same position) may decrease venous return.
F: Fluid and salt (volume expansion)
Maintaining an adequate plasma volume is crucial. Patients should drink five to eight 8-ounce glasses (1.25 to 2.5 L) of water or other fluid per day. Many elderly people do not take in this much. The patient should have at least 1 glass or cup of fluid with meals and at least twice at other times of each day to obtain 1 L/day.
Salt intake should be between 150 and 250 mmol of sodium (10 to 20 g of salt) per day. Sodium helps with retention of ingested fluids and should be maximized if tolerated. However, caution should be exercised in patients who have severe refractory supine hypertension, uncontrolled hypertension, or comorbidities characterized by insterstitial edema (eg, heart failure, liver failure). Some patients are very sensitive to sodium supplementation and can fine-tune their orthostatic control with salt alone. If salting food is not desired, prepared soups, pretzels, potato chips, and 0.5- or 1.0-g salt tablets can be an option.
Patients need to maintain a high-potassium diet, as the high sodium intake combined with fludrocortisone promotes potassium loss. Fruits (especially bananas) and vegetables have high potassium content.
The combination of fludrocortisone and a high-salt diet can also cause sustained supine hypertension, which can be minimized by the interventions noted in Table 2.
Appropriate salt supplementation and fluid intake leading to an adequate volume expansion can be verified by checking the 24-hour urinary sodium content: patients who excrete less than 170 mmol can be treated with 1 to 2 g of supplemental sodium three times a day.38
- Sjostrand T. The regulation of the blood distribution in man. Acta Physiol Scand 1952; 26:312–327.
- Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med 1977; 296:293–297.
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46:1470.
- Gehrking JA, Hines SM, Benrud-Larson LM, Opher-Gehrking TL, Low PA. What is the minimum duration of head-up tilt necessary to detect orthostatic hypotension? Clin Auton Res 2005; 15:71–75.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 2005; 30:173–178.
- Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med 2002; 162:2369–2374.
- Mader SL, Josephson KR, Rubenstein LZ. Low prevalence of postural hypotension among community-dwelling elderly. JAMA 1987; 258:1511–1514.
- Sandroni P, Ahlskog JE, Fealey RD, Low PA. Autonomic involvement in extrapyramidal and cerebellar disorders. Clin Auton Res 1991; 1:147–155.
- Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med 1994; 33:321–325.
- Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation 1987; 75:340–346.
- Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med 1999; 159:273–280.
- Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med 1993; 329:611–615.
- Denq JC, Opfer-Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res 1997; 7:321–326.
- Sjostrand T. Volume and distribution of blood and their significance in regulating the circulation. Physiol Rev 1953; 33:202–228.
- Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol 1972; 32:213–220.
- Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004; 14:167–175.
- Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation 2000; 101:504–509.
- Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med 2002; 112:355–360.
- Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water [letter]. Lancet 1999; 353:723.
- MacLean AR, Allen EV. Orthostatic hypotension and orthostatic tachycardia: treatment with the “head-up” bed. JAMA 1940; 115:2162–2167.
- Ector H, Reybrouck T, Heidbüchel H, Gewillig M, Van de Werf F. Tilt training: a new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pacing Clin Electrophysiol 1998; 21:193–196.
- Bouvette CM, McPhee BR, Opfer-Gehrking TL, Low PA. Role of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentation. Mayo Clin Proc 1996; 71:847–853.
- Ten Harkel AD, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci (Lond) 1994; 87:553–558.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med 1993; 95:38–48.
- Fouad-Tarazi FM, Okabe M, Goren H. Alpha sympathomimetic treatment of autonomic insufficiency with orthostatic hypotension. Am J Med 1995; 99:604–610.
- Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets 2007; 7:63–70.
- Axelrod FB, Goldberg JD, Rolnitzky L, et al. Fludrocortisone in patients with familial dysautonomia—assessing effect on clinical parameters and gene expression. Clin Auton Res 2005; 15:284–291.
- Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. N Engl J Med 1979; 301:68–73.
- van Lieshout JJ, Ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res 2000; 10:35–42.
- Hussain RM, McIntosh SJ, Lawson J, Kenny RA. Fludrocortisone in the treatment of hypotensive disorders in the elderly. Heart 1996; 76:507–509.
- Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry 2003; 74:1294–1298.
- Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006; 63:513–518.
- Lipsitz LA, Pluchino FC, Wei JY, Rowe JW. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis 1986; 39:619–630.
- Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol 1999; 84:121–130.
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol 2001; 85:420–426.
- El-Sayed H, Hainsworth R. Salt supplementation increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart 1996; 75:134–140.
- Sjostrand T. The regulation of the blood distribution in man. Acta Physiol Scand 1952; 26:312–327.
- Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med 1977; 296:293–297.
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46:1470.
- Gehrking JA, Hines SM, Benrud-Larson LM, Opher-Gehrking TL, Low PA. What is the minimum duration of head-up tilt necessary to detect orthostatic hypotension? Clin Auton Res 2005; 15:71–75.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 2005; 30:173–178.
- Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med 2002; 162:2369–2374.
- Mader SL, Josephson KR, Rubenstein LZ. Low prevalence of postural hypotension among community-dwelling elderly. JAMA 1987; 258:1511–1514.
- Sandroni P, Ahlskog JE, Fealey RD, Low PA. Autonomic involvement in extrapyramidal and cerebellar disorders. Clin Auton Res 1991; 1:147–155.
- Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med 1994; 33:321–325.
- Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation 1987; 75:340–346.
- Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med 1999; 159:273–280.
- Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med 1993; 329:611–615.
- Denq JC, Opfer-Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res 1997; 7:321–326.
- Sjostrand T. Volume and distribution of blood and their significance in regulating the circulation. Physiol Rev 1953; 33:202–228.
- Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol 1972; 32:213–220.
- Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004; 14:167–175.
- Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation 2000; 101:504–509.
- Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med 2002; 112:355–360.
- Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water [letter]. Lancet 1999; 353:723.
- MacLean AR, Allen EV. Orthostatic hypotension and orthostatic tachycardia: treatment with the “head-up” bed. JAMA 1940; 115:2162–2167.
- Ector H, Reybrouck T, Heidbüchel H, Gewillig M, Van de Werf F. Tilt training: a new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pacing Clin Electrophysiol 1998; 21:193–196.
- Bouvette CM, McPhee BR, Opfer-Gehrking TL, Low PA. Role of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentation. Mayo Clin Proc 1996; 71:847–853.
- Ten Harkel AD, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci (Lond) 1994; 87:553–558.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med 1993; 95:38–48.
- Fouad-Tarazi FM, Okabe M, Goren H. Alpha sympathomimetic treatment of autonomic insufficiency with orthostatic hypotension. Am J Med 1995; 99:604–610.
- Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets 2007; 7:63–70.
- Axelrod FB, Goldberg JD, Rolnitzky L, et al. Fludrocortisone in patients with familial dysautonomia—assessing effect on clinical parameters and gene expression. Clin Auton Res 2005; 15:284–291.
- Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. N Engl J Med 1979; 301:68–73.
- van Lieshout JJ, Ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res 2000; 10:35–42.
- Hussain RM, McIntosh SJ, Lawson J, Kenny RA. Fludrocortisone in the treatment of hypotensive disorders in the elderly. Heart 1996; 76:507–509.
- Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry 2003; 74:1294–1298.
- Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006; 63:513–518.
- Lipsitz LA, Pluchino FC, Wei JY, Rowe JW. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis 1986; 39:619–630.
- Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol 1999; 84:121–130.
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol 2001; 85:420–426.
- El-Sayed H, Hainsworth R. Salt supplementation increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart 1996; 75:134–140.
KEY POINTS
- Treatment is directed at increasing blood volume, decreasing venous pooling, and increasing vasoconstriction while minimizing supine hypertension.
- Patient education and nondrug strategies alone can be effective in mild cases. Examples: consuming extra fluids and salt, wearing an abdominal binder, drinking boluses of water, raising the head of the bed, and performing countermaneuvers and physical activity.
- Moderate and severe cases require additional drug treatment. Pyridostigmine (Mestinon) is helpful in moderate cases. Fludrocortisone (Florinef) and midodrine (ProAmatine) are indicated in more severe cases.