User login
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
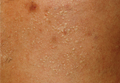
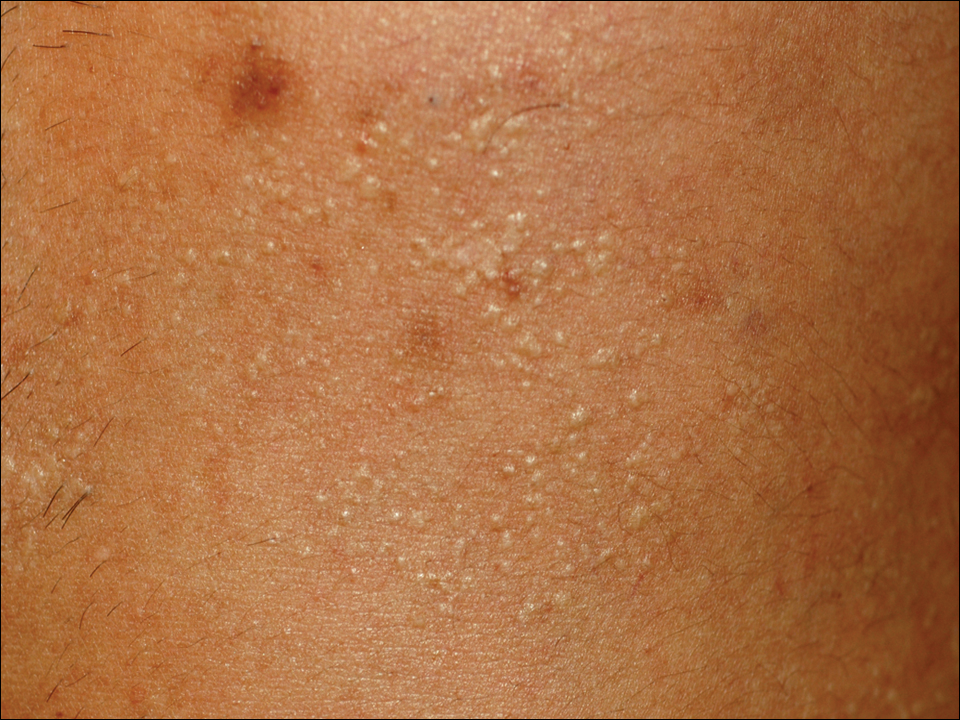
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.
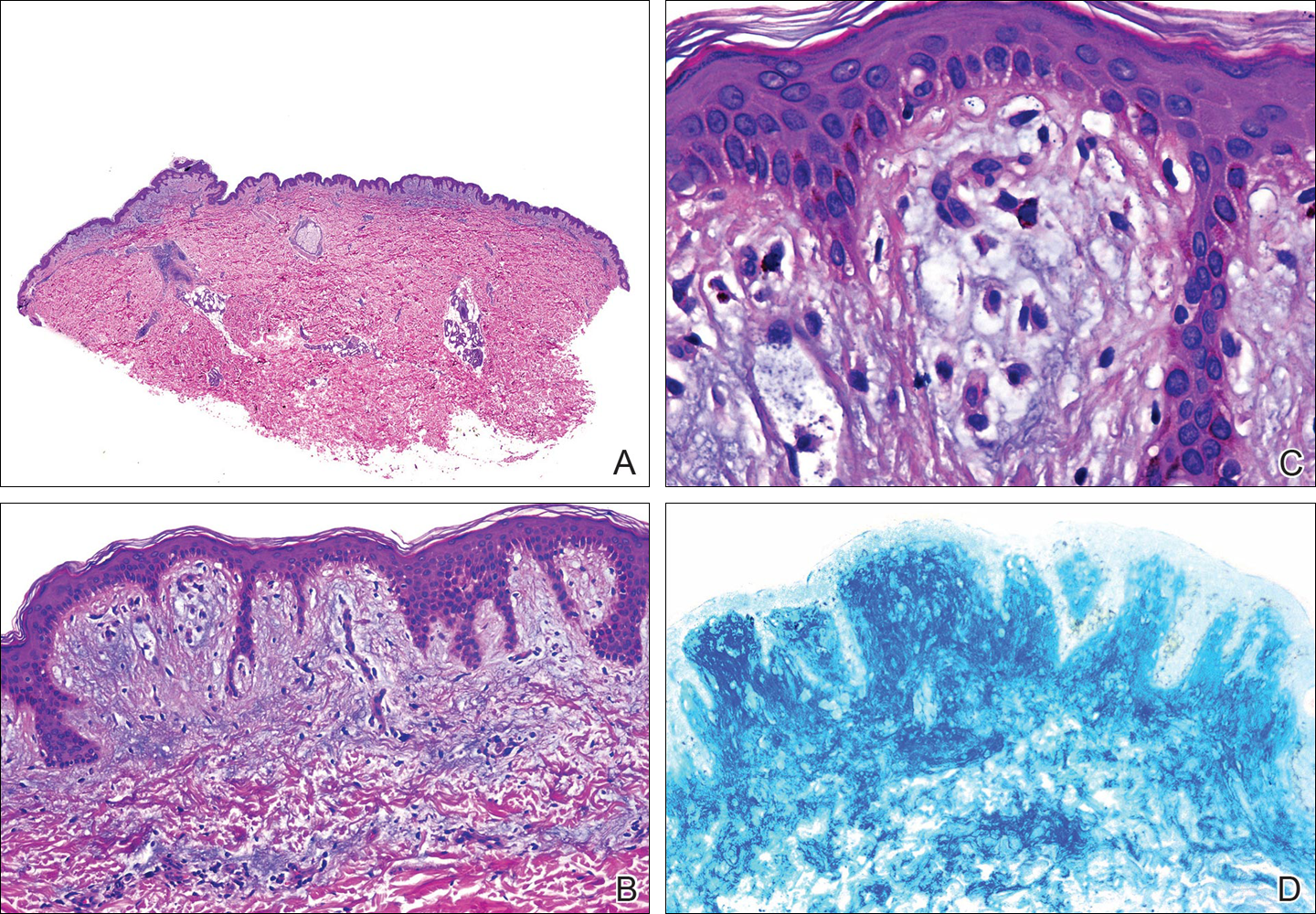
Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.
Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Practice Points
- Focal mucinosis is a histopathologic finding that may be seen in different cutaneous disorders. It is an exceptional histopathologic finding that has rarely been described in herpes zoster scars.
- In most cases, focal mucinosis is just a histopathologic finding with no therapeutic consequences.