User login
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
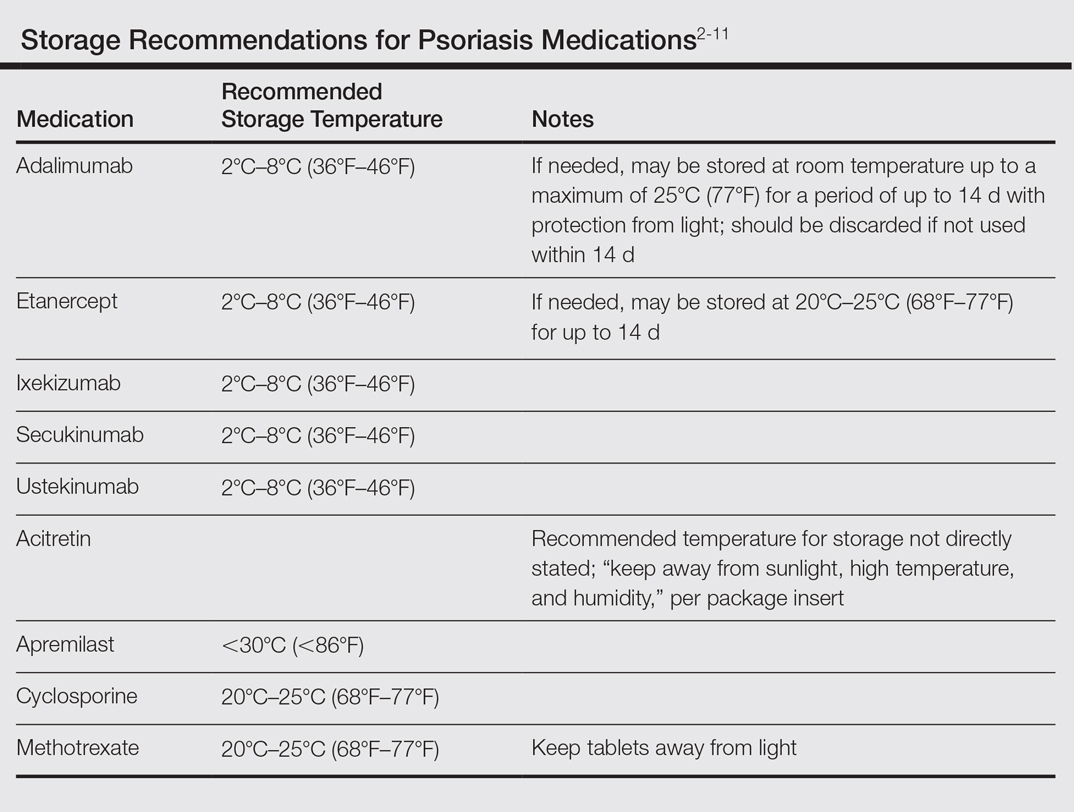
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Practice Points
- Establishing goals of treatment with each patient is a critical step in treating the patient rather than the diagnosis.
- A good social history can reveal job-related impact of disease and potential logistical roadblocks to treatment.
- Efficacy must be weighed against the burden of logistical constraints for each patient; potential issues include difficulty complying with follow-up visits, access to laboratory monitoring, exposure to pathogens, and adequacy of medication transport and storage.