User login
Transgender and Gender Diverse Health Care in the US Military: What Dermatologists Need to Know
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
Practice Points
- Transgender and gender diverse (TGD) health care is multidisciplinary, and both military and civilian dermatologists can serve an important role.
- Although dermatologists do not directly perform gender-affirming surgeries or hormone management, there are a number of dermatologic procedures and medical interventions that can enhance a TGD person’s desired appearance.
- Dermatologists also can help manage possible adverse effects from gender-affirming interventions.
The Burden of Skin Cancer in the Military Health System, 2017-2022
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.
Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.
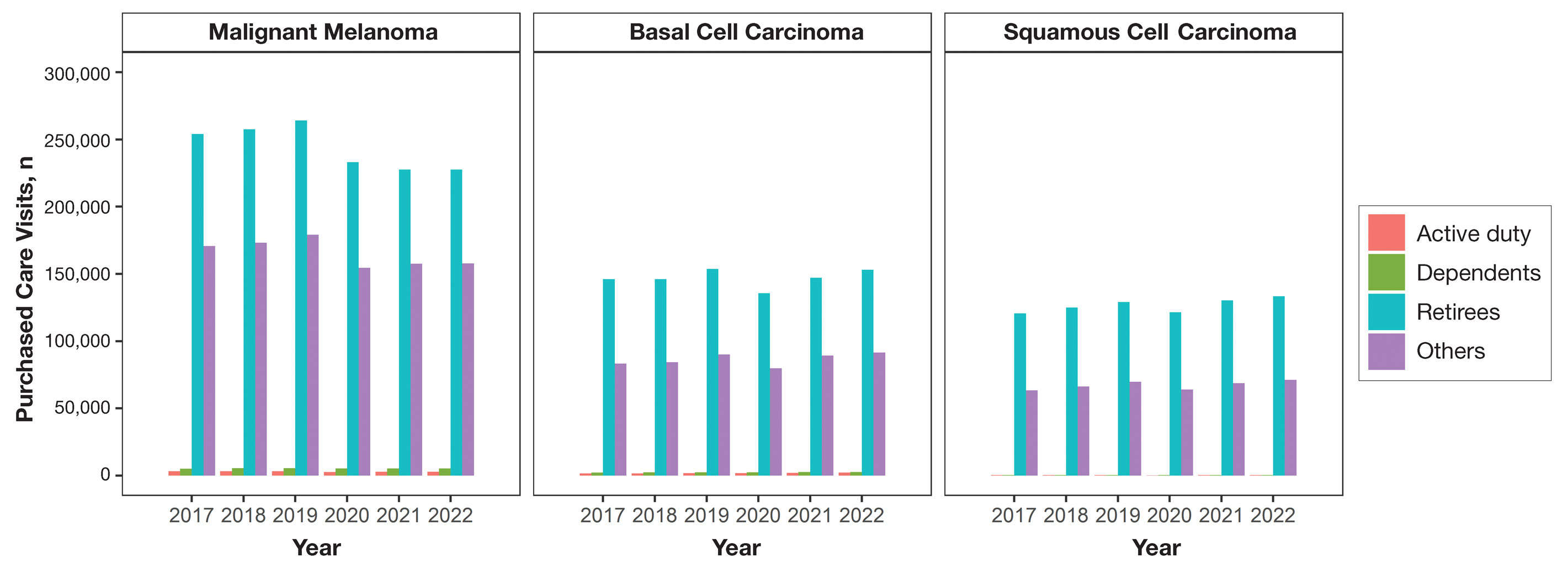
When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.
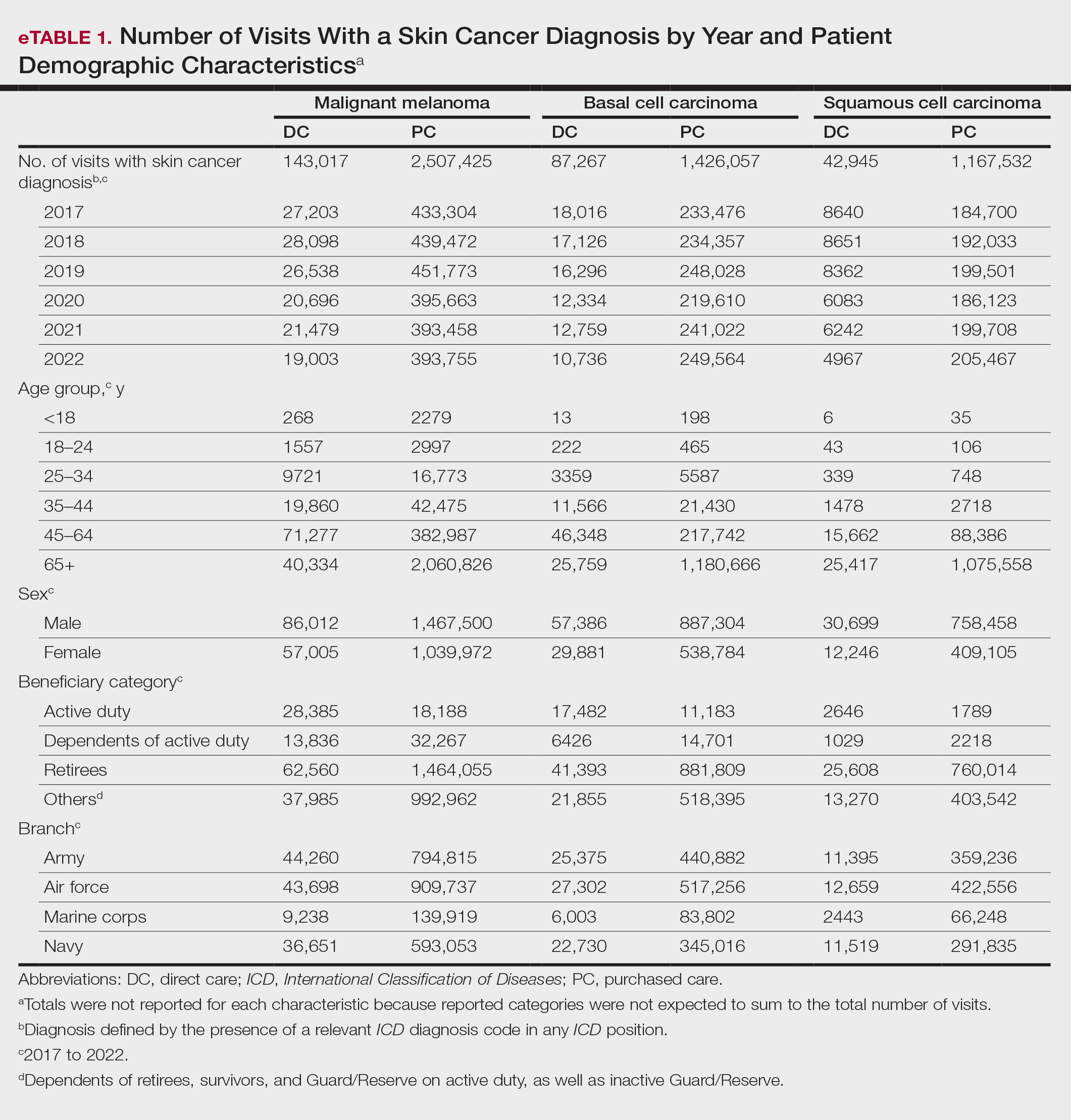
The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.
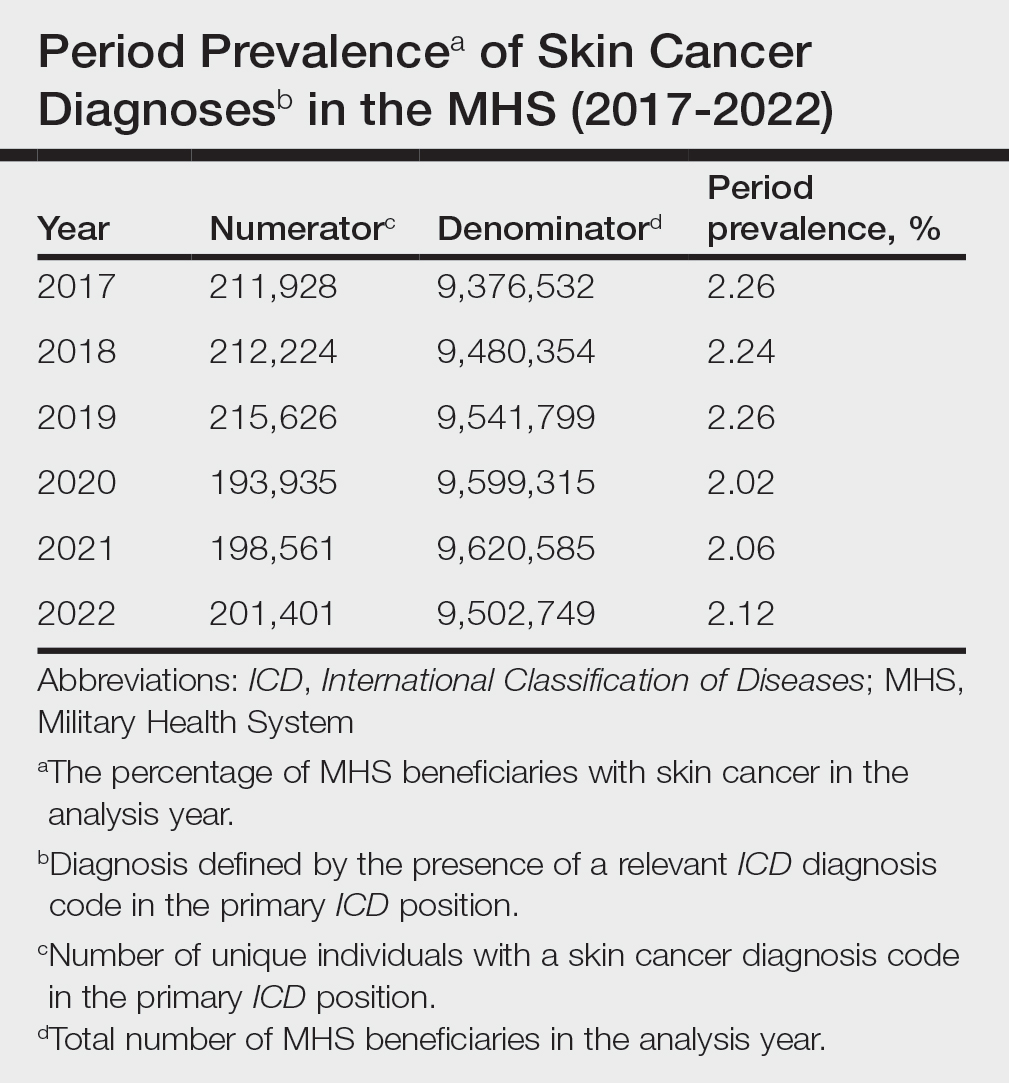
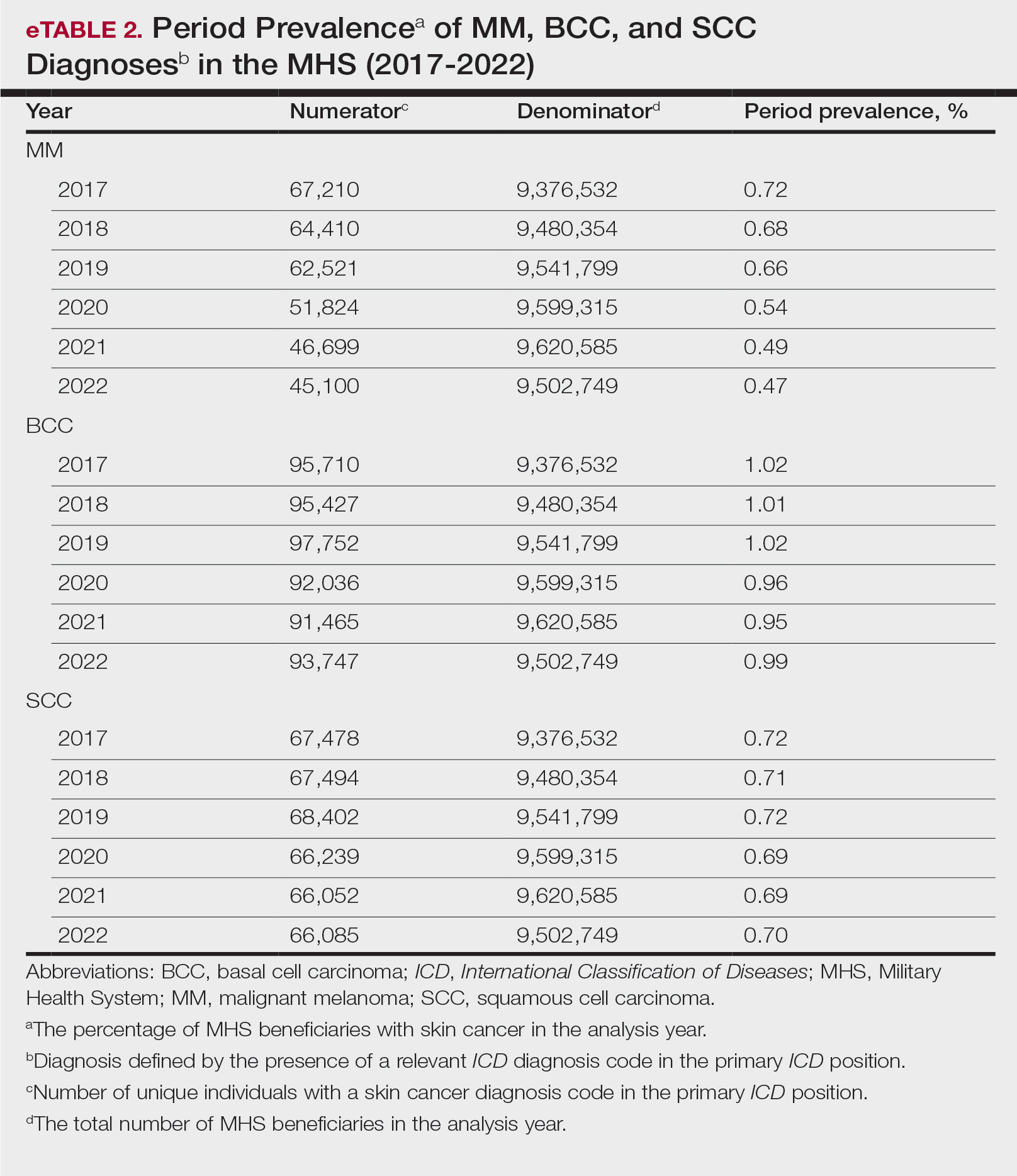
Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
PRACTICE POINTS
- Study data showed an overall decreasing prevalence of skin cancer in the Military Health System (MHS) from 2019 to 2021, possibly attributable to underdiagnosis resulting from the COVID-19 pandemic. Providers should be mindful of this trend when screening patients who have experienced interruptions in care.
- An overall increased prevalence of skin cancer was noted in the military beneficiary population compared with publicly available civilian data—and thus this diagnosis should be given special consideration within this population.
Wound Healing: Cellular Review With Specific Attention to Postamputation Care
Restoring skin integrity and balance after injury is vital for survival, serving as a crucial defense mechanism against potential infections by preventing the entry of harmful pathogens. Moreover, proper healing is essential for restoring normal tissue function, allowing damaged tissues to repair and, in an ideal scenario, regenerate. Timely healing helps reduce the risk for complications, such as chronic wounds, which could lead to more severe issues if left untreated. Additionally, pain relief often is associated with effective wound healing as inflammatory responses diminish during the repair process.
The immune system plays a pivotal role in wound healing, influencing various repair mechanisms and ultimately determining the extent of scarring. Although inflammation is present throughout the repair response, recent studies have challenged the conventional belief of an inverse correlation between the intensity of inflammation and regenerative capacity. Inflammatory signals were found to be crucial for timely repair and fundamental processes in regeneration, possibly presenting a paradigm shift in the understanding of immunology.1-4 The complexities of wound healing are exemplified when evaluating and treating postamputation wounds. To address such a task, one needs a firm understanding of the science behind healing wounds and what can go wrong along the way.
Phases of Wound Healing
Wound healing is a complex process that involves a series of sequential yet overlapping phases, including hemostasis/inflammation, proliferation, and remodeling.
Hemostasis/Inflammation—The initial stage of wound healing involves hemostasis, in which the primary objective is to prevent blood loss and initiate inflammation. Platelets arrive at the wound site, forming a provisional clot that is crucial for subsequent healing phases.4-6 Platelets halt bleeding as well as act as a medium for cell migration and adhesion; they also are a source of growth factors and proinflammatory cytokines that herald the inflammatory response.4-7
Inflammation is characterized by the infiltration of immune cells, particularly neutrophils and macrophages. Neutrophils act as the first line of defense, clearing debris and preventing infection. Macrophages follow, phagocytizing apoptotic cells and releasing growth factors such as tumor necrosis factor α, vascular endothelial growth factor, and matrix metalloprotease 9, which stimulate the next phase.4-6,8 Typically, the hemostasis and inflammatory phase starts approximately 6 to 8 hours after wound origin and lasts 3 to 4 days.4,6,7
Proliferation—Following hemostasis and inflammation, the wound transitions into the proliferation phase, which is marked by the development of granulation tissue—a dynamic amalgamation of fibroblasts, endothelial cells, and inflammatory cells.1,4-8 Fibroblasts play a central role in synthesizing collagen, the primary structural protein in connective tissue. They also orchestrate synthesis of vitronectin, fibronectin, fibrin, and tenascin.4-6,8 Simultaneously, angiogenesis takes place, involving the creation of new blood vessels to supply essential nutrients and oxygen to the healing tissue.4,7,9 Growth factors such as transforming growth factor β and vascular endothelial growth factor coordinate cellular activities and foster tissue repair.4-6,8 The proliferation phase extends over days to weeks, laying the groundwork for subsequent tissue restructuring.
Remodeling—The final stage of wound healing is remodeling, an extended process that may persist for several months or, in some cases, years. Throughout this phase, the initially deposited collagen, predominantly type III collagen, undergoes transformation into mature type I collagen.4-6,8 This transformation is critical for reinstating the tissue’s strength and functionality. The balance between collagen synthesis and degradation is delicate, regulated by matrix metalloproteinases and inhibitors of metalloproteinases.4-8 Fibroblasts, myofibroblasts, and other cells coordinate this intricate process of tissue reorganization.4-7
The eventual outcome of the remodeling phase determines the appearance and functionality of the healed tissue. Any disruption in this phase can lead to complications, such as chronic wounds and hypertrophic scars/keloids.4-6 These abnormal healing processes are characterized by localized inflammation, heightened fibroblast function, and excessive accumulation of the extracellular matrix.4-8
Molecular Mechanisms
Comprehensive investigations—both in vivo and in vitro—have explored the intricate molecular mechanisms involved in heightened wound healing. Transforming growth factor β takes center stage as a crucial factor, prompting the transformation of fibroblasts into myofibroblasts and contributing to the deposition of extracellular matrix.2,4-8,10 Transforming growth factor β activates non-Smad signaling pathways, such as MAPK (mitogen-activated protein kinase) and PI3K (phosphoinositide 3-kinase), influencing processes associated with fibrosis.5,11 Furthermore, microRNAs play a pivotal role in posttranscriptional regulation, influencing both transforming growth factor β signaling and fibroblast behavior.12-16
The involvement of prostaglandins is crucial in wound healing. Prostaglandin E2 plays a notable role and is positively correlated with the rate of wound healing.5 The cyclooxygenase pathway, pivotal for prostaglandin synthesis, becomes a target for inflammation control.4,5,10 Although aspirin and nonsteroidal anti-inflammatory drugs commonly are employed, their impact on wound healing remains controversial, as inhibition of cyclooxygenase may disrupt normal repair processes.5,17,18
Wound healing exhibits variations depending on age. Fetal skin regeneration is marked by the restoration of normal dermal architecture, including adnexal structures, nerves, vessels, and muscle.4-6 The distinctive characteristics of fetal wound healing include a unique profile of growth factors, a diminished inflammatory response, reduced biomechanical stress, and a distinct extracellular matrix composition.19 These factors contribute to a lower propensity for scar formation compared to the healing processes observed in adults. Fetal and adult wound healing differ fundamentally in their extracellular matrix composition, inflammatory cells, and cytokine levels.4-6,19 Adult wounds feature myofibroblasts, which are absent in fetal wounds, contributing to heightened mechanical tension.5 Delving deeper into the biochemical basis of fetal wound healing holds promise for mitigating scar formation in adults.
Takeaways From Other Species
Much of the biochemical knowledge of wound healing, especially regenerative wound healing, is known from other species. Geckos provide a unique model for studying regenerative repair in tails and nonregenerative healing in limbs after amputation. Scar-free wound healing is characterized by rapid wound closure, delayed blood vessel development, and collagen deposition, which contrasts with the hypervascular granulation tissue seen in scarring wounds.20 Scar-free wound healing and regeneration are intrinsic properties of the lizard tail and are unaffected by the location or method of detachment.21
Compared to amphibians with extraordinary regenerative capacity, data suggest the lack of regenerative capacity in mammals may come from a desynchronization of the fine-tuned interplay of progenitor cells such as blastema and differentiated cells.22,23 In mice, the response to amputation is specific to the level: cutting through the distal third of the terminal phalanx elicits a regeneration response, yielding a new digit tip resembling the lost one, while an amputation through the distal third of the intermediate phalanx triggers a wound healing and scarring response.24
Wound Healing Following Limb Amputation
Limb amputation represents a profound change in an individual’s life, impacting daily activities and overall well-being. There are many causes of amputation, but the most common include cardiovascular diseases, diabetes mellitus, cancer, and trauma.25-27 Trauma represents a relatively common cause within the US Military due to the overall young population as well as inherent risks of uniformed service.25,27 Advances in protective gear and combat casualty care have led to an increased number of individuals surviving with extremity injuries requiring amputation, particularly among younger service members, with a subgroup experiencing multiple amputations.27-29
Numerous factors play a crucial role in the healing and function of postamputation wounds. The level of amputation is a key determinant influencing both functional outcomes and the healing process. Achieving a balance between preserving function and removing damaged tissue is essential. A study investigating cardiac function and oxygen consumption in 25 patients with peripheral vascular disease found higher-level amputations resulted in decreased walking speed and cadence, along with increased oxygen consumption per meter walked.30
Selecting the appropriate amputation level is vital to optimize functional outcomes without compromising wound healing. Successful prosthetic limb fitting depends largely on the length of the residual stump to support the body load and suspend the prosthesis. For long bone amputations, maintaining at least 12-cm clearance above the knee joint in transfemoral amputees and 10-cm below the knee joint in transtibial amputees is critical for maximizing functional outcomes.31
Surgical technique also is paramount. The goal is to minimize the risk for pressure ulcers by avoiding bony spurs and muscle imbalances. Shaping the muscle and residual limb is essential for proper prosthesis fitting. Attention to neurovascular structures, such as burying nerve ends to prevent neuropathic pain during prosthesis wear, is crucial.32 In extremity amputations, surgeons often resort to free flap transfer techniques for stump reconstruction. In a study of 31 patients with severe lower extremity injuries undergoing various amputations, the use of latissimus dorsi myocutaneous flaps, alone or in combination with serratus anterior muscle flaps, resulted in fewer instances of deep ulceration and allowed for earlier prosthesis wear.33
Addressing Barriers to Wound Healing
Multiple barriers to successful wound healing are encountered in the amputee population. Amputations from trauma have a less-controlled initiation, which carries with it a higher risk for infection, poor wound healing, and other complications.
Infection—Infection often is one of the first hurdles encountered in postamputation wound healing. Critical first steps in infection prevention include thorough cleaning of soiled traumatic wounds and appropriate tissue debridement coupled with scrupulous sterile technique and postoperative monitoring for signs and symptoms of infection.
In a retrospective study of 223 combat-related major lower extremity amputations (initial and revision) between 2009 and 2015, the use of intrawound antibiotic powder at the time of closure demonstrated a 13% absolute risk reduction in deep infection rates, which was particularly notable in revision amputations, with a number needed to treat of 8 for initial amputations and 4 for revision amputations on previously infected limbs.34 Intra-operative antibiotic powder may represent a cheap and easy consideration for this special population of amputees. Postamputation antibiotic prophylaxis for infection prevention is an area of controversy. For nontraumatic infections, data suggest antibiotic prophylaxis may not decrease infection rates in these patients.35,36
Interestingly, a study by Azarbal et al37 aimed to investigate the correlation between nasal methicillin-resistant Staphylococcus aureus (MRSA) colonization and other patient factors with wound occurrence following major lower extremity amputation. The study found MRSA colonization was associated with higher rates of overall wound occurrence as well as wound occurrence due to wound infection. These data suggest nasal MRSA eradication may improve postoperative wound outcomes after major lower extremity amputation.37
Dressing Choice—The dressing chosen for a residual limb also is of paramount importance following amputation. The personalized and dynamic management of postamputation wounds and skin involves achieving optimal healing through a dressing that sustains appropriate moisture levels, addresses edema, helps prevent contractures, and safeguards the limb.38 From the start, using negative pressure wound dressings after surgical amputation can decrease wound-related complications.39
Topical oxygen therapy following amputation also shows promise. In a retrospective case series by Kalliainen et al,40 topical oxygen therapy applied to 58 wounds in 32 patients over 9 months demonstrated positive outcomes in promoting wound healing, with 38 wounds (66%) healing completely with the use of topical oxygen. Minimal complications and no detrimental effects were observed.40
Current recommendations suggest that non–weight-bearing removable rigid dressings are the superior postoperative management for transtibial amputations compared to soft dressings, offering benefits such as faster healing, reduced limb edema, earlier ambulation, preparatory shaping for prosthetic use, and prevention of knee flexion contractures.41-46 Similarly, adding a silicone liner following amputation significantly reduced the duration of prosthetic rehabilitation compared with a conventional soft dressing program in one study (P<.05).47
Specifically targeting wound edema, a case series by Hoskins et al48 investigated the impact of prostheses with vacuum-assisted suspension on the size of residual limb wounds in individuals with transtibial amputation. Well-fitting sockets with vacuum-assisted suspension did not impede wound healing, and the results suggest the potential for continued prosthesis use during the healing process.48 However, a study by Johannesson et al49 compared the outcomes of transtibial amputation patients using a vacuum-formed rigid dressing and a conventional rigid plaster dressing, finding no significant differences in wound healing, time to prosthetic fitting, or functional outcomes with the prosthesis between the 2 groups. When comparing elastic bandaging, pneumatic prosthesis, and temporary prosthesis on postoperative stump management, temporary prosthesis led to a decrease in stump volume, quicker transition to a permanent prosthesis, and improved quality of life compared with elastic bandaging and pneumatic prosthetics.50
The type of material in dressings may contribute to utility in amputation wounds. Keratin-based wound dressings show promise for wound healing, especially in recalcitrant vascular wounds.51 There also are numerous proprietary wound dressings available for patients, at least one of which has particularly thorough data. In a retrospective study of more than 2 million lower extremity wounds across 644 institutions, a proprietary bioactive human skin allograft (TheraSkin [LifeNet Health]) demonstrated higher healing rates, greater percentage area reductions, lower amputations, reduced recidivism, higher treatment completion, and fewer medical transfers compared with standard of care alone.52
Postamputation Dermatologic Concerns
After the postamputation wound heals, a notable concern is the prevalence of skin diseases affecting residual limbs. The stump site in amputees, marked by a delicate cutaneous landscape vulnerable to skin diseases, faces challenges arising from amputation-induced damage to various structures.53
When integrated into a prosthesis socket, the altered skin must acclimate to a humid environment and endure forces for which it is not well suited, especially during movement.53 Amputation remarkably alters normal tissue perfusion, which can lead to aberrant blood and lymphatic circulation in residual limbs.27,53 This compromised skin, often associated with a history of vascular disease, diabetes mellitus, or malignancy, becomes immunocompromised, heightening the risk for dermatologic issues such as inflammation, infection, and malignancies.53 Unlike the resilient volar skin on palms and soles, stump skin lacks adaptation to withstand the compressive forces generated during ambulation, sometimes leading to skin disease and pain that result in abandonment of the prosthesis.53,54 Mechanical forces on the skin, especially in active patients eager to resume pre-injury lifestyles, contribute to skin breakdown. The dynamic nature of the residual limb, including muscle atrophy, gait changes, and weight fluctuations, complicates the prosthetic fitting process. Prosthesis abandonment remains a challenge, despite modern technologic advancements.
The occurrence of heterotopic ossification (extraskeletal bone formation) is another notable issue in military amputees.27,55-57 Poor prosthetic fit can lead to skin degradation, necessitating further surgery to address mispositioned bone formations. Orthopedic monitoring supplemented by appropriate imaging studies can benefit postamputation patients by detecting and preventing heterotopic ossification in its early stages.
Dermatologic issues, especially among lower limb amputees, are noteworthy, with a substantial percentage experiencing complications related to socket prosthetics, such as heat, sweating, sores, and skin irritation. Up to 41% of patients are seen regularly for a secondary skin disorder following amputation.58 As one might expect, persistent wounds, blisters, ulcers, and abscesses are some of the most typical cutaneous abnormalities affecting residual limbs with prostheses.27,58 More rare skin conditions also are documented in residual limbs, including cutaneous granuloma, verrucous carcinoma, bullous pemphigoid, and angiodermatitis.27,59-61
Treatments offered in the dermatology clinic often are similar to patients who have not had an amputation. For instance, hyperhidrosis can be treated with prescription antiperspirant, topical aluminum chloride, topical glycopyrronium, botulinum toxin, and iontophoresis, which can greatly decrease skin irritation and malodor. Subcutaneous neurotoxins such as botulinum toxin are especially useful for hyperhidrosis following amputation because a single treatment can last 3 to 6 months, whereas topicals must be applied multiple times per day and can be inherently irritating to the skin.27,62 Furthermore, ablative fractional resurfacing lasers also can help stimulate new collagen growth, increase skin mobility on residual limbs, smooth jagged scars, and aid prosthetic fitting.27,63 Perforated prosthetic liners also may be useful to address issues such as excessive sweating, demonstrating improvements in skin health, reduced sweating problems, and potential avoidance of surgical interventions.64
When comorbid skin conditions are at bay, preventive measures for excessive wound healing necessitate early recognition and timely intervention for residual limbs. Preventive techniques encompass the use of silicone gel sheeting, hypoallergenic microporous tape, and intralesional steroid injections.
Psychological Concerns—An overarching issue following amputation is the psychological toll the process imposes on the patient. Psychological concerns, including anxiety and depression, present additional challenges impacting residual limb hygiene and prosthetic maintenance. Chronic wounds are devastating to patients. These patients consistently express feeling ostracized from their community and anxious about unemployment, leaking fluid, or odor from the wound, as well as other social stigmata.62 Depression and anxiety can hinder a patient’s ability to care for their wound and make them more susceptible to the myriad issues that can ensue.
Recent Developments in Wound Healing
Wound healing is ripe for innovation that could assuage ailments that impact patients following amputation. A 2022 study by Abu El Hawa et al65 illustrated advanced progression in wound healing for patients taking statins, even though the statin group had increased age and number of comorbidities compared with patients not taking statins.
Nasseri and Sharifi66 showed the potential of antimicrobial peptides—small proteins with cationic charges and amphipathic structures exhibiting electrostatic interaction with microbial cell membranes—in promoting wound healing, particularly defensins and cathelicidin LL-37.They also discussed innovative delivery systems, such as nanoparticles and electrospun fibrous scaffolds, highlighting their potential as possibly more effective therapeutics than antibiotics, especially in the context of diabetic wound closure.66 Aimed at increased angiogenesis in the proliferative phase, there is evidence that N-acetylcysteine can increase amputation stump perfusion with the goal of better long-term wound healing and more efficient scar formation.67
Stem cell therapy, particularly employing cells from the human amniotic membrane, represents an auspicious avenue for antifibrotic treatment. Amniotic epithelial cells and amniotic mesenchymal cells, with their self-renewal and multilineage differentiation capabilities, exhibit anti-inflammatory and antifibrotic properties.4,5 A study by Dong et al68 aimed to assess the efficacy of cell therapy, particularly differentiated progenitor cell–based graft transplantation or autologous stem cell injection, in treating refractory skin injuries such as nonrevascularizable critical limb ischemic ulcers, venous leg ulcers, and diabetic lower limb ulcers. The findings demonstrated cell therapy effectively reduced the size of ulcers, improved wound closure rates, and decreased major amputation rates compared with standard therapy. Of note, cell therapy had limited impact on alleviating pain in patients with critical limb ischemia-related cutaneous ulcers.68
Final Thoughts
Wound care following amputation is a multidisciplinary endeavor, necessitating collaboration between many health care professionals. Dermatologists play a crucial role in providing routine care as well as addressing wound healing and related skin issues among amputation patients. As the field progresses, dermatologists are well positioned to make notable contributions and ensure enhanced outcomes, resulting in a better quality of life for patients facing the challenges of limb amputation and prosthetic use.
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525-549. doi:10.1146/annurev.cellbio.24.110707.175336
- Eming SA, Hammerschmidt M, Krieg T, et al. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20:517-527. doi:10.1016/j.semcdb.2009.04.009
- Eming SA. Evolution of immune pathways in regeneration and repair: recent concepts and translational perspectives. Semin Immunol. 2014;26:275-276. doi:10.1016/j.smim.2014.09.001
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 4th edition. Elsevier; 2018.
- Wang PH, Huang BS, Horng HC, et al. Wound healing. J Chin Med Assoc JCMA. 2018;81:94-101. doi:10.1016/j.jcma.2017.11.002
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528-1542. doi:10.1177/147323000903700531
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321. doi:10.1038/nature07039
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi:10.1126/scitranslmed.3009337
- Eming SA, Brachvogel B, Odorisio T, et al. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115-170. doi:10.1016/j.proghi.2007.06.001
- Janis JE, Harrison B. Wound healing: part I. basic science. Plast Reconstr Surg. 2016;138(3 suppl):9S-17S. doi:10.1097/PRS.0000000000002773
- Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics. part I: the molecular basis of scar formation. J Am Acad Dermatol. 2012;66:1-10; quiz 11-12. doi:10.1016/j.jaad.2011.05.055
- Kwan P, Ding J, Tredget EE. MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS One. 2015;10:e0123054. doi:10.1371/journal.pone.0123054
- Ben W, Yang Y, Yuan J, et al. Human papillomavirus 16 E6 modulates the expression of host microRNAs in cervical cancer. Taiwan J Obstet Gynecol. 2015;54:364-370. doi:10.1016/j.tjog.2014.06.007
- Yu EH, Tu HF, Wu CH, et al. MicroRNA-21 promotes perineural invasion and impacts survival in patients with oral carcinoma. J Chin Med Assoc JCMA. 2017;80:383-388. doi:10.1016/j.jcma.2017.01.003
- Wen KC, Sung PL, Yen MS, et al. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J Obstet Gynecol. 2013;52:465-469. doi:10.1016/j.tjog.2013.10.002
- Babalola O, Mamalis A, Lev-Tov H, et al. The role of microRNAs in skin fibrosis. Arch Dermatol Res. 2013;305:763-776. doi:10.1007/s00403-013-1410-1
- Hofer M, Hoferová Z, Falk M. Pharmacological modulation of radiation damage. does it exist a chance for other substances than hematopoietic growth factors and cytokines? Int J Mol Sci. 2017;18:1385. doi:10.3390/ijms18071385
- Darby IA, Weller CD. Aspirin treatment for chronic wounds: potential beneficial and inhibitory effects. Wound Repair Regen. 2017;25:7-12. doi:10.1111/wrr.12502
- Khalid KA, Nawi AFM, Zulkifli N, et al. Aging and wound healing of the skin: a review of clinical and pathophysiological hallmarks. Life. 2022;12:2142. doi:10.3390/life12122142
- Peacock HM, Gilbert EAB, Vickaryous MK. Scar‐free cutaneous wound healing in the leopard gecko, Eublepharis macularius. J Anat. 2015;227:596-610. doi:10.1111/joa.12368
- Delorme SL, Lungu IM, Vickaryous MK. Scar‐free wound healing and regeneration following tail loss in the leopard gecko, Eublepharis macularius. Anat Rec. 2012;295:1575-1595. doi:10.1002/ar.22490
- Brunauer R, Xia IG, Asrar SN, et al. Aging delays epimorphic regeneration in mice. J Gerontol Ser A Biol Sci Med Sci. 2021;76:1726-1733. doi:10.1093/gerona/glab131
- Dolan CP, Yang TJ, Zimmel K, et al. Epimorphic regeneration of the mouse digit tip is finite. Stem Cell Res Ther. 2022;13:62. doi:10.1186/s13287-022-02741-2
- Simkin J, Han M, Yu L, et al. The mouse digit tip: from wound healing to regeneration. Methods Mol Biol Clifton NJ. 2013;1037:419-435. doi:10.1007/978-1-62703-505-7_24
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429. doi:10.1016/j.apmr.2007.11.005
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663. doi:10.1016/j.apmr.2004.09.003
- Lannan FM, Meyerle JH. The dermatologist’s role in amputee skin care. Cutis. 2019;103:86-90.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777. doi:10.1016/j.injury.2009.02.014
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385. doi:10.1682/jrrd.2009.03.0023
- Pinzur MS, Gold J, Schwartz D, et al. Energy demands for walking in dysvascular amputees as related to the level of amputation. Orthopedics. 1992;15:1033-1036; discussion 1036-1037. doi:10.3928/0147-7447-19920901-07
- Robinson V, Sansam K, Hirst L, et al. Major lower limb amputation–what, why and how to achieve the best results. Orthop Trauma. 2010;24:276-285. doi:10.1016/j.mporth.2010.03.017
- Lu S, Wang C, Zhong W, et al. Amputation stump revision using a free sural neurocutaneous perforator flap. Ann Plast Surg. 2016;76:83-87. doi:10.1097/SAP.0000000000000211
- Kim SW, Jeon SB, Hwang KT, et al. Coverage of amputation stumps using a latissimus dorsi flap with a serratus anterior muscle flap: a comparative study. Ann Plast Surg. 2016;76:88-93. doi:10.1097/SAP.0000000000000220
- Pavey GJ, Formby PM, Hoyt BW, et al. Intrawound antibiotic powder decreases frequency of deep infection and severity of heterotopic ossification in combat lower extremity amputations. Clin Orthop. 2019;477:802-810. doi:10.1007/s11999.0000000000000090
- Dunkel N, Belaieff W, Assal M, et al. Wound dehiscence and stump infection after lower limb amputation: risk factors and association with antibiotic use. J Orthop Sci Off J Jpn Orthop Assoc. 2012;17:588-594. doi:10.1007/s00776-012-0245-5
- Rubin G, Orbach H, Rinott M, et al. The use of prophylactic antibiotics in treatment of fingertip amputation: a randomized prospective trial. Am J Emerg Med. 2015;33:645-647. doi:10.1016/j.ajem.2015.02.002
- Azarbal AF, Harris S, Mitchell EL, et al. Nasal methicillin-resistant Staphylococcus aureus colonization is associated with increased wound occurrence after major lower extremity amputation. J Vasc Surg. 2015;62:401-405. doi:10.1016/j.jvs.2015.02.052
- Kwasniewski M, Mitchel D. Post amputation skin and wound care. Phys Med Rehabil Clin N Am. 2022;33:857-870. doi:10.1016/j.pmr.2022.06.010
- Chang H, Maldonado TS, Rockman CB, et al. Closed incision negative pressure wound therapy may decrease wound complications in major lower extremity amputations. J Vasc Surg. 2021;73:1041-1047. doi:10.1016/j.jvs.2020.07.061
- Kalliainen LK, Gordillo GM, Schlanger R, et al. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiol Off J Int Soc Pathophysiol. 2003;9:81-87. doi:10.1016/s0928-4680(02)00079-2
- Reichmann JP, Stevens PM, Rheinstein J, et al. Removable rigid dressings for postoperative management of transtibial amputations: a review of published evidence. PM R. 2018;10:516-523. doi:10.1016/j.pmrj.2017.10.002
- MacLean N, Fick GH. The effect of semirigid dressings on below-knee amputations. Phys Ther. 1994;74:668-673. doi:10.1093/ptj/74.7.668
- Koonalinthip N, Sukthongsa A, Janchai S. Comparison of removable rigid dressing and elastic bandage for residual limb maturation in transtibial amputees: a randomized controlled trial. Arch Phys Med Rehabil. 2020;101:1683-1688. doi:10.1016/j.apmr.2020.05.009
- Taylor L, Cavenett S, Stepien JM, et al. Removable rigid dressings: a retrospective case-note audit to determine the validity of post-amputation application. Prosthet Orthot Int. 2008;32:223-230. doi:10.1080/03093640802016795
- Sumpio B, Shine SR, Mahler D, et al. A comparison of immediate postoperative rigid and soft dressings for below-knee amputations. Ann Vasc Surg. 2013;27:774-780. doi:10.1016/j.avsg.2013.03.007
- van Velzen AD, Nederhand MJ, Emmelot CH, et al. Early treatment of trans-tibial amputees: retrospective analysis of early fitting and elastic bandaging. Prosthet Orthot Int. 2005;29:3-12. doi:10.1080/17461550500069588
- Chin T, Toda M. Results of prosthetic rehabilitation on managing transtibial vascular amputation with silicone liner after wound closure. J Int Med Res. 2016;44:957-967. doi:10.1177/0300060516647554
- Hoskins RD, Sutton EE, Kinor D, et al. Using vacuum-assisted suspension to manage residual limb wounds in persons with transtibial amputation: a case series. Prosthet Orthot Int. 2014;38:68-74. doi:10.1177/0309364613487547
- Johannesson A, Larsson GU, Oberg T, et al. Comparison of vacuum-formed removable rigid dressing with conventional rigid dressing after transtibial amputation: similar outcome in a randomized controlled trial involving 27 patients. Acta Orthop. 2008;79:361-369. doi:10.1080/17453670710015265
- Alsancak S, Köse SK, Altınkaynak H. Effect of elastic bandaging and prosthesis on the decrease in stump volume. Acta Orthop Traumatol Turc. 2011;45:14-22. doi:10.3944/AOTT.2011.2365
- Than MP, Smith RA, Hammond C, et al. Keratin-based wound care products for treatment of resistant vascular wounds. J Clin Aesthetic Dermatol. 2012;5:31-35.
- Gurtner GC, Garcia AD, Bakewell K, et al. A retrospective matched‐cohort study of 3994 lower extremity wounds of multiple etiologies across 644 institutions comparing a bioactive human skin allograft, TheraSkin, plus standard of care, to standard of care alone. Int Wound J. 2020;17:55-64. doi:10.1111/iwj.13231
- Buikema KES, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677. doi:10.1016/j.clindermatol.2014.04.015
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286. doi:10.1001/archdermatol.2012.3004
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486. doi:10.2106/JBJS.F.00412
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30. doi:10.1097/BOT.0000000000000666
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237. doi:10.1097/BOT.0b013e3182a53130
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump. Clin Exp Dermatol. 2011;36:560-561. doi:10.1111/j.1365-2230.2011.04037.x
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb. Int J Dermatol. 2012;51:75-78. doi:10.1111/j.1365-4632.2011.04906.x
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatologica. 1991;182:193-195. doi:10.1159/000247782
- Campanati A, Diotallevi F, Radi G, et al. Efficacy and safety of botulinum toxin B in focal hyperhidrosis: a narrative review. Toxins. 2023;15:147. doi:10.3390/toxins15020147
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193. doi:10.1001/jamadermatol.2013.7761
- McGrath M, McCarthy J, Gallego A, et al. The influence of perforated prosthetic liners on residual limb wound healing: a case report. Can Prosthet Orthot J. 2019;2:32723. doi:10.33137/cpoj.v2i1.32723
- Abu El Hawa AA, Klein D, Bekeny JC, et al. The impact of statins on wound healing: an ally in treating the highly comorbid patient. J Wound Care. 2022;31(suppl 2):S36-S41. doi:10.12968/jowc.2022.31.Sup2.S36
- Nasseri S, Sharifi M. Therapeutic potential of antimicrobial peptides for wound healing. Int J Pept Res Ther. 2022;28:38. doi:10.1007/s10989-021-10350-5
- Lee JV, Engel C, Tay S, et al. N-Acetyl-Cysteine treatment after lower extremity amputation improves areas of perfusion defect and wound healing outcomes. J Vasc Surg. 2021;73:39-40. doi:10.1016/j.jvs.2020.12.025
- Dong Y, Yang Q, Sun X. Comprehensive analysis of cell therapy on chronic skin wound healing: a meta-analysis. Hum Gene Ther. 2021;32:787-795. doi:10.1089/hum.2020.275
Restoring skin integrity and balance after injury is vital for survival, serving as a crucial defense mechanism against potential infections by preventing the entry of harmful pathogens. Moreover, proper healing is essential for restoring normal tissue function, allowing damaged tissues to repair and, in an ideal scenario, regenerate. Timely healing helps reduce the risk for complications, such as chronic wounds, which could lead to more severe issues if left untreated. Additionally, pain relief often is associated with effective wound healing as inflammatory responses diminish during the repair process.
The immune system plays a pivotal role in wound healing, influencing various repair mechanisms and ultimately determining the extent of scarring. Although inflammation is present throughout the repair response, recent studies have challenged the conventional belief of an inverse correlation between the intensity of inflammation and regenerative capacity. Inflammatory signals were found to be crucial for timely repair and fundamental processes in regeneration, possibly presenting a paradigm shift in the understanding of immunology.1-4 The complexities of wound healing are exemplified when evaluating and treating postamputation wounds. To address such a task, one needs a firm understanding of the science behind healing wounds and what can go wrong along the way.
Phases of Wound Healing
Wound healing is a complex process that involves a series of sequential yet overlapping phases, including hemostasis/inflammation, proliferation, and remodeling.
Hemostasis/Inflammation—The initial stage of wound healing involves hemostasis, in which the primary objective is to prevent blood loss and initiate inflammation. Platelets arrive at the wound site, forming a provisional clot that is crucial for subsequent healing phases.4-6 Platelets halt bleeding as well as act as a medium for cell migration and adhesion; they also are a source of growth factors and proinflammatory cytokines that herald the inflammatory response.4-7
Inflammation is characterized by the infiltration of immune cells, particularly neutrophils and macrophages. Neutrophils act as the first line of defense, clearing debris and preventing infection. Macrophages follow, phagocytizing apoptotic cells and releasing growth factors such as tumor necrosis factor α, vascular endothelial growth factor, and matrix metalloprotease 9, which stimulate the next phase.4-6,8 Typically, the hemostasis and inflammatory phase starts approximately 6 to 8 hours after wound origin and lasts 3 to 4 days.4,6,7
Proliferation—Following hemostasis and inflammation, the wound transitions into the proliferation phase, which is marked by the development of granulation tissue—a dynamic amalgamation of fibroblasts, endothelial cells, and inflammatory cells.1,4-8 Fibroblasts play a central role in synthesizing collagen, the primary structural protein in connective tissue. They also orchestrate synthesis of vitronectin, fibronectin, fibrin, and tenascin.4-6,8 Simultaneously, angiogenesis takes place, involving the creation of new blood vessels to supply essential nutrients and oxygen to the healing tissue.4,7,9 Growth factors such as transforming growth factor β and vascular endothelial growth factor coordinate cellular activities and foster tissue repair.4-6,8 The proliferation phase extends over days to weeks, laying the groundwork for subsequent tissue restructuring.
Remodeling—The final stage of wound healing is remodeling, an extended process that may persist for several months or, in some cases, years. Throughout this phase, the initially deposited collagen, predominantly type III collagen, undergoes transformation into mature type I collagen.4-6,8 This transformation is critical for reinstating the tissue’s strength and functionality. The balance between collagen synthesis and degradation is delicate, regulated by matrix metalloproteinases and inhibitors of metalloproteinases.4-8 Fibroblasts, myofibroblasts, and other cells coordinate this intricate process of tissue reorganization.4-7
The eventual outcome of the remodeling phase determines the appearance and functionality of the healed tissue. Any disruption in this phase can lead to complications, such as chronic wounds and hypertrophic scars/keloids.4-6 These abnormal healing processes are characterized by localized inflammation, heightened fibroblast function, and excessive accumulation of the extracellular matrix.4-8
Molecular Mechanisms
Comprehensive investigations—both in vivo and in vitro—have explored the intricate molecular mechanisms involved in heightened wound healing. Transforming growth factor β takes center stage as a crucial factor, prompting the transformation of fibroblasts into myofibroblasts and contributing to the deposition of extracellular matrix.2,4-8,10 Transforming growth factor β activates non-Smad signaling pathways, such as MAPK (mitogen-activated protein kinase) and PI3K (phosphoinositide 3-kinase), influencing processes associated with fibrosis.5,11 Furthermore, microRNAs play a pivotal role in posttranscriptional regulation, influencing both transforming growth factor β signaling and fibroblast behavior.12-16
The involvement of prostaglandins is crucial in wound healing. Prostaglandin E2 plays a notable role and is positively correlated with the rate of wound healing.5 The cyclooxygenase pathway, pivotal for prostaglandin synthesis, becomes a target for inflammation control.4,5,10 Although aspirin and nonsteroidal anti-inflammatory drugs commonly are employed, their impact on wound healing remains controversial, as inhibition of cyclooxygenase may disrupt normal repair processes.5,17,18
Wound healing exhibits variations depending on age. Fetal skin regeneration is marked by the restoration of normal dermal architecture, including adnexal structures, nerves, vessels, and muscle.4-6 The distinctive characteristics of fetal wound healing include a unique profile of growth factors, a diminished inflammatory response, reduced biomechanical stress, and a distinct extracellular matrix composition.19 These factors contribute to a lower propensity for scar formation compared to the healing processes observed in adults. Fetal and adult wound healing differ fundamentally in their extracellular matrix composition, inflammatory cells, and cytokine levels.4-6,19 Adult wounds feature myofibroblasts, which are absent in fetal wounds, contributing to heightened mechanical tension.5 Delving deeper into the biochemical basis of fetal wound healing holds promise for mitigating scar formation in adults.
Takeaways From Other Species
Much of the biochemical knowledge of wound healing, especially regenerative wound healing, is known from other species. Geckos provide a unique model for studying regenerative repair in tails and nonregenerative healing in limbs after amputation. Scar-free wound healing is characterized by rapid wound closure, delayed blood vessel development, and collagen deposition, which contrasts with the hypervascular granulation tissue seen in scarring wounds.20 Scar-free wound healing and regeneration are intrinsic properties of the lizard tail and are unaffected by the location or method of detachment.21
Compared to amphibians with extraordinary regenerative capacity, data suggest the lack of regenerative capacity in mammals may come from a desynchronization of the fine-tuned interplay of progenitor cells such as blastema and differentiated cells.22,23 In mice, the response to amputation is specific to the level: cutting through the distal third of the terminal phalanx elicits a regeneration response, yielding a new digit tip resembling the lost one, while an amputation through the distal third of the intermediate phalanx triggers a wound healing and scarring response.24
Wound Healing Following Limb Amputation
Limb amputation represents a profound change in an individual’s life, impacting daily activities and overall well-being. There are many causes of amputation, but the most common include cardiovascular diseases, diabetes mellitus, cancer, and trauma.25-27 Trauma represents a relatively common cause within the US Military due to the overall young population as well as inherent risks of uniformed service.25,27 Advances in protective gear and combat casualty care have led to an increased number of individuals surviving with extremity injuries requiring amputation, particularly among younger service members, with a subgroup experiencing multiple amputations.27-29
Numerous factors play a crucial role in the healing and function of postamputation wounds. The level of amputation is a key determinant influencing both functional outcomes and the healing process. Achieving a balance between preserving function and removing damaged tissue is essential. A study investigating cardiac function and oxygen consumption in 25 patients with peripheral vascular disease found higher-level amputations resulted in decreased walking speed and cadence, along with increased oxygen consumption per meter walked.30
Selecting the appropriate amputation level is vital to optimize functional outcomes without compromising wound healing. Successful prosthetic limb fitting depends largely on the length of the residual stump to support the body load and suspend the prosthesis. For long bone amputations, maintaining at least 12-cm clearance above the knee joint in transfemoral amputees and 10-cm below the knee joint in transtibial amputees is critical for maximizing functional outcomes.31
Surgical technique also is paramount. The goal is to minimize the risk for pressure ulcers by avoiding bony spurs and muscle imbalances. Shaping the muscle and residual limb is essential for proper prosthesis fitting. Attention to neurovascular structures, such as burying nerve ends to prevent neuropathic pain during prosthesis wear, is crucial.32 In extremity amputations, surgeons often resort to free flap transfer techniques for stump reconstruction. In a study of 31 patients with severe lower extremity injuries undergoing various amputations, the use of latissimus dorsi myocutaneous flaps, alone or in combination with serratus anterior muscle flaps, resulted in fewer instances of deep ulceration and allowed for earlier prosthesis wear.33
Addressing Barriers to Wound Healing
Multiple barriers to successful wound healing are encountered in the amputee population. Amputations from trauma have a less-controlled initiation, which carries with it a higher risk for infection, poor wound healing, and other complications.
Infection—Infection often is one of the first hurdles encountered in postamputation wound healing. Critical first steps in infection prevention include thorough cleaning of soiled traumatic wounds and appropriate tissue debridement coupled with scrupulous sterile technique and postoperative monitoring for signs and symptoms of infection.
In a retrospective study of 223 combat-related major lower extremity amputations (initial and revision) between 2009 and 2015, the use of intrawound antibiotic powder at the time of closure demonstrated a 13% absolute risk reduction in deep infection rates, which was particularly notable in revision amputations, with a number needed to treat of 8 for initial amputations and 4 for revision amputations on previously infected limbs.34 Intra-operative antibiotic powder may represent a cheap and easy consideration for this special population of amputees. Postamputation antibiotic prophylaxis for infection prevention is an area of controversy. For nontraumatic infections, data suggest antibiotic prophylaxis may not decrease infection rates in these patients.35,36
Interestingly, a study by Azarbal et al37 aimed to investigate the correlation between nasal methicillin-resistant Staphylococcus aureus (MRSA) colonization and other patient factors with wound occurrence following major lower extremity amputation. The study found MRSA colonization was associated with higher rates of overall wound occurrence as well as wound occurrence due to wound infection. These data suggest nasal MRSA eradication may improve postoperative wound outcomes after major lower extremity amputation.37
Dressing Choice—The dressing chosen for a residual limb also is of paramount importance following amputation. The personalized and dynamic management of postamputation wounds and skin involves achieving optimal healing through a dressing that sustains appropriate moisture levels, addresses edema, helps prevent contractures, and safeguards the limb.38 From the start, using negative pressure wound dressings after surgical amputation can decrease wound-related complications.39
Topical oxygen therapy following amputation also shows promise. In a retrospective case series by Kalliainen et al,40 topical oxygen therapy applied to 58 wounds in 32 patients over 9 months demonstrated positive outcomes in promoting wound healing, with 38 wounds (66%) healing completely with the use of topical oxygen. Minimal complications and no detrimental effects were observed.40
Current recommendations suggest that non–weight-bearing removable rigid dressings are the superior postoperative management for transtibial amputations compared to soft dressings, offering benefits such as faster healing, reduced limb edema, earlier ambulation, preparatory shaping for prosthetic use, and prevention of knee flexion contractures.41-46 Similarly, adding a silicone liner following amputation significantly reduced the duration of prosthetic rehabilitation compared with a conventional soft dressing program in one study (P<.05).47
Specifically targeting wound edema, a case series by Hoskins et al48 investigated the impact of prostheses with vacuum-assisted suspension on the size of residual limb wounds in individuals with transtibial amputation. Well-fitting sockets with vacuum-assisted suspension did not impede wound healing, and the results suggest the potential for continued prosthesis use during the healing process.48 However, a study by Johannesson et al49 compared the outcomes of transtibial amputation patients using a vacuum-formed rigid dressing and a conventional rigid plaster dressing, finding no significant differences in wound healing, time to prosthetic fitting, or functional outcomes with the prosthesis between the 2 groups. When comparing elastic bandaging, pneumatic prosthesis, and temporary prosthesis on postoperative stump management, temporary prosthesis led to a decrease in stump volume, quicker transition to a permanent prosthesis, and improved quality of life compared with elastic bandaging and pneumatic prosthetics.50
The type of material in dressings may contribute to utility in amputation wounds. Keratin-based wound dressings show promise for wound healing, especially in recalcitrant vascular wounds.51 There also are numerous proprietary wound dressings available for patients, at least one of which has particularly thorough data. In a retrospective study of more than 2 million lower extremity wounds across 644 institutions, a proprietary bioactive human skin allograft (TheraSkin [LifeNet Health]) demonstrated higher healing rates, greater percentage area reductions, lower amputations, reduced recidivism, higher treatment completion, and fewer medical transfers compared with standard of care alone.52
Postamputation Dermatologic Concerns
After the postamputation wound heals, a notable concern is the prevalence of skin diseases affecting residual limbs. The stump site in amputees, marked by a delicate cutaneous landscape vulnerable to skin diseases, faces challenges arising from amputation-induced damage to various structures.53
When integrated into a prosthesis socket, the altered skin must acclimate to a humid environment and endure forces for which it is not well suited, especially during movement.53 Amputation remarkably alters normal tissue perfusion, which can lead to aberrant blood and lymphatic circulation in residual limbs.27,53 This compromised skin, often associated with a history of vascular disease, diabetes mellitus, or malignancy, becomes immunocompromised, heightening the risk for dermatologic issues such as inflammation, infection, and malignancies.53 Unlike the resilient volar skin on palms and soles, stump skin lacks adaptation to withstand the compressive forces generated during ambulation, sometimes leading to skin disease and pain that result in abandonment of the prosthesis.53,54 Mechanical forces on the skin, especially in active patients eager to resume pre-injury lifestyles, contribute to skin breakdown. The dynamic nature of the residual limb, including muscle atrophy, gait changes, and weight fluctuations, complicates the prosthetic fitting process. Prosthesis abandonment remains a challenge, despite modern technologic advancements.
The occurrence of heterotopic ossification (extraskeletal bone formation) is another notable issue in military amputees.27,55-57 Poor prosthetic fit can lead to skin degradation, necessitating further surgery to address mispositioned bone formations. Orthopedic monitoring supplemented by appropriate imaging studies can benefit postamputation patients by detecting and preventing heterotopic ossification in its early stages.
Dermatologic issues, especially among lower limb amputees, are noteworthy, with a substantial percentage experiencing complications related to socket prosthetics, such as heat, sweating, sores, and skin irritation. Up to 41% of patients are seen regularly for a secondary skin disorder following amputation.58 As one might expect, persistent wounds, blisters, ulcers, and abscesses are some of the most typical cutaneous abnormalities affecting residual limbs with prostheses.27,58 More rare skin conditions also are documented in residual limbs, including cutaneous granuloma, verrucous carcinoma, bullous pemphigoid, and angiodermatitis.27,59-61
Treatments offered in the dermatology clinic often are similar to patients who have not had an amputation. For instance, hyperhidrosis can be treated with prescription antiperspirant, topical aluminum chloride, topical glycopyrronium, botulinum toxin, and iontophoresis, which can greatly decrease skin irritation and malodor. Subcutaneous neurotoxins such as botulinum toxin are especially useful for hyperhidrosis following amputation because a single treatment can last 3 to 6 months, whereas topicals must be applied multiple times per day and can be inherently irritating to the skin.27,62 Furthermore, ablative fractional resurfacing lasers also can help stimulate new collagen growth, increase skin mobility on residual limbs, smooth jagged scars, and aid prosthetic fitting.27,63 Perforated prosthetic liners also may be useful to address issues such as excessive sweating, demonstrating improvements in skin health, reduced sweating problems, and potential avoidance of surgical interventions.64
When comorbid skin conditions are at bay, preventive measures for excessive wound healing necessitate early recognition and timely intervention for residual limbs. Preventive techniques encompass the use of silicone gel sheeting, hypoallergenic microporous tape, and intralesional steroid injections.
Psychological Concerns—An overarching issue following amputation is the psychological toll the process imposes on the patient. Psychological concerns, including anxiety and depression, present additional challenges impacting residual limb hygiene and prosthetic maintenance. Chronic wounds are devastating to patients. These patients consistently express feeling ostracized from their community and anxious about unemployment, leaking fluid, or odor from the wound, as well as other social stigmata.62 Depression and anxiety can hinder a patient’s ability to care for their wound and make them more susceptible to the myriad issues that can ensue.
Recent Developments in Wound Healing
Wound healing is ripe for innovation that could assuage ailments that impact patients following amputation. A 2022 study by Abu El Hawa et al65 illustrated advanced progression in wound healing for patients taking statins, even though the statin group had increased age and number of comorbidities compared with patients not taking statins.
Nasseri and Sharifi66 showed the potential of antimicrobial peptides—small proteins with cationic charges and amphipathic structures exhibiting electrostatic interaction with microbial cell membranes—in promoting wound healing, particularly defensins and cathelicidin LL-37.They also discussed innovative delivery systems, such as nanoparticles and electrospun fibrous scaffolds, highlighting their potential as possibly more effective therapeutics than antibiotics, especially in the context of diabetic wound closure.66 Aimed at increased angiogenesis in the proliferative phase, there is evidence that N-acetylcysteine can increase amputation stump perfusion with the goal of better long-term wound healing and more efficient scar formation.67
Stem cell therapy, particularly employing cells from the human amniotic membrane, represents an auspicious avenue for antifibrotic treatment. Amniotic epithelial cells and amniotic mesenchymal cells, with their self-renewal and multilineage differentiation capabilities, exhibit anti-inflammatory and antifibrotic properties.4,5 A study by Dong et al68 aimed to assess the efficacy of cell therapy, particularly differentiated progenitor cell–based graft transplantation or autologous stem cell injection, in treating refractory skin injuries such as nonrevascularizable critical limb ischemic ulcers, venous leg ulcers, and diabetic lower limb ulcers. The findings demonstrated cell therapy effectively reduced the size of ulcers, improved wound closure rates, and decreased major amputation rates compared with standard therapy. Of note, cell therapy had limited impact on alleviating pain in patients with critical limb ischemia-related cutaneous ulcers.68
Final Thoughts
Wound care following amputation is a multidisciplinary endeavor, necessitating collaboration between many health care professionals. Dermatologists play a crucial role in providing routine care as well as addressing wound healing and related skin issues among amputation patients. As the field progresses, dermatologists are well positioned to make notable contributions and ensure enhanced outcomes, resulting in a better quality of life for patients facing the challenges of limb amputation and prosthetic use.
Restoring skin integrity and balance after injury is vital for survival, serving as a crucial defense mechanism against potential infections by preventing the entry of harmful pathogens. Moreover, proper healing is essential for restoring normal tissue function, allowing damaged tissues to repair and, in an ideal scenario, regenerate. Timely healing helps reduce the risk for complications, such as chronic wounds, which could lead to more severe issues if left untreated. Additionally, pain relief often is associated with effective wound healing as inflammatory responses diminish during the repair process.
The immune system plays a pivotal role in wound healing, influencing various repair mechanisms and ultimately determining the extent of scarring. Although inflammation is present throughout the repair response, recent studies have challenged the conventional belief of an inverse correlation between the intensity of inflammation and regenerative capacity. Inflammatory signals were found to be crucial for timely repair and fundamental processes in regeneration, possibly presenting a paradigm shift in the understanding of immunology.1-4 The complexities of wound healing are exemplified when evaluating and treating postamputation wounds. To address such a task, one needs a firm understanding of the science behind healing wounds and what can go wrong along the way.
Phases of Wound Healing
Wound healing is a complex process that involves a series of sequential yet overlapping phases, including hemostasis/inflammation, proliferation, and remodeling.
Hemostasis/Inflammation—The initial stage of wound healing involves hemostasis, in which the primary objective is to prevent blood loss and initiate inflammation. Platelets arrive at the wound site, forming a provisional clot that is crucial for subsequent healing phases.4-6 Platelets halt bleeding as well as act as a medium for cell migration and adhesion; they also are a source of growth factors and proinflammatory cytokines that herald the inflammatory response.4-7
Inflammation is characterized by the infiltration of immune cells, particularly neutrophils and macrophages. Neutrophils act as the first line of defense, clearing debris and preventing infection. Macrophages follow, phagocytizing apoptotic cells and releasing growth factors such as tumor necrosis factor α, vascular endothelial growth factor, and matrix metalloprotease 9, which stimulate the next phase.4-6,8 Typically, the hemostasis and inflammatory phase starts approximately 6 to 8 hours after wound origin and lasts 3 to 4 days.4,6,7
Proliferation—Following hemostasis and inflammation, the wound transitions into the proliferation phase, which is marked by the development of granulation tissue—a dynamic amalgamation of fibroblasts, endothelial cells, and inflammatory cells.1,4-8 Fibroblasts play a central role in synthesizing collagen, the primary structural protein in connective tissue. They also orchestrate synthesis of vitronectin, fibronectin, fibrin, and tenascin.4-6,8 Simultaneously, angiogenesis takes place, involving the creation of new blood vessels to supply essential nutrients and oxygen to the healing tissue.4,7,9 Growth factors such as transforming growth factor β and vascular endothelial growth factor coordinate cellular activities and foster tissue repair.4-6,8 The proliferation phase extends over days to weeks, laying the groundwork for subsequent tissue restructuring.
Remodeling—The final stage of wound healing is remodeling, an extended process that may persist for several months or, in some cases, years. Throughout this phase, the initially deposited collagen, predominantly type III collagen, undergoes transformation into mature type I collagen.4-6,8 This transformation is critical for reinstating the tissue’s strength and functionality. The balance between collagen synthesis and degradation is delicate, regulated by matrix metalloproteinases and inhibitors of metalloproteinases.4-8 Fibroblasts, myofibroblasts, and other cells coordinate this intricate process of tissue reorganization.4-7
The eventual outcome of the remodeling phase determines the appearance and functionality of the healed tissue. Any disruption in this phase can lead to complications, such as chronic wounds and hypertrophic scars/keloids.4-6 These abnormal healing processes are characterized by localized inflammation, heightened fibroblast function, and excessive accumulation of the extracellular matrix.4-8
Molecular Mechanisms
Comprehensive investigations—both in vivo and in vitro—have explored the intricate molecular mechanisms involved in heightened wound healing. Transforming growth factor β takes center stage as a crucial factor, prompting the transformation of fibroblasts into myofibroblasts and contributing to the deposition of extracellular matrix.2,4-8,10 Transforming growth factor β activates non-Smad signaling pathways, such as MAPK (mitogen-activated protein kinase) and PI3K (phosphoinositide 3-kinase), influencing processes associated with fibrosis.5,11 Furthermore, microRNAs play a pivotal role in posttranscriptional regulation, influencing both transforming growth factor β signaling and fibroblast behavior.12-16
The involvement of prostaglandins is crucial in wound healing. Prostaglandin E2 plays a notable role and is positively correlated with the rate of wound healing.5 The cyclooxygenase pathway, pivotal for prostaglandin synthesis, becomes a target for inflammation control.4,5,10 Although aspirin and nonsteroidal anti-inflammatory drugs commonly are employed, their impact on wound healing remains controversial, as inhibition of cyclooxygenase may disrupt normal repair processes.5,17,18
Wound healing exhibits variations depending on age. Fetal skin regeneration is marked by the restoration of normal dermal architecture, including adnexal structures, nerves, vessels, and muscle.4-6 The distinctive characteristics of fetal wound healing include a unique profile of growth factors, a diminished inflammatory response, reduced biomechanical stress, and a distinct extracellular matrix composition.19 These factors contribute to a lower propensity for scar formation compared to the healing processes observed in adults. Fetal and adult wound healing differ fundamentally in their extracellular matrix composition, inflammatory cells, and cytokine levels.4-6,19 Adult wounds feature myofibroblasts, which are absent in fetal wounds, contributing to heightened mechanical tension.5 Delving deeper into the biochemical basis of fetal wound healing holds promise for mitigating scar formation in adults.
Takeaways From Other Species
Much of the biochemical knowledge of wound healing, especially regenerative wound healing, is known from other species. Geckos provide a unique model for studying regenerative repair in tails and nonregenerative healing in limbs after amputation. Scar-free wound healing is characterized by rapid wound closure, delayed blood vessel development, and collagen deposition, which contrasts with the hypervascular granulation tissue seen in scarring wounds.20 Scar-free wound healing and regeneration are intrinsic properties of the lizard tail and are unaffected by the location or method of detachment.21
Compared to amphibians with extraordinary regenerative capacity, data suggest the lack of regenerative capacity in mammals may come from a desynchronization of the fine-tuned interplay of progenitor cells such as blastema and differentiated cells.22,23 In mice, the response to amputation is specific to the level: cutting through the distal third of the terminal phalanx elicits a regeneration response, yielding a new digit tip resembling the lost one, while an amputation through the distal third of the intermediate phalanx triggers a wound healing and scarring response.24
Wound Healing Following Limb Amputation
Limb amputation represents a profound change in an individual’s life, impacting daily activities and overall well-being. There are many causes of amputation, but the most common include cardiovascular diseases, diabetes mellitus, cancer, and trauma.25-27 Trauma represents a relatively common cause within the US Military due to the overall young population as well as inherent risks of uniformed service.25,27 Advances in protective gear and combat casualty care have led to an increased number of individuals surviving with extremity injuries requiring amputation, particularly among younger service members, with a subgroup experiencing multiple amputations.27-29
Numerous factors play a crucial role in the healing and function of postamputation wounds. The level of amputation is a key determinant influencing both functional outcomes and the healing process. Achieving a balance between preserving function and removing damaged tissue is essential. A study investigating cardiac function and oxygen consumption in 25 patients with peripheral vascular disease found higher-level amputations resulted in decreased walking speed and cadence, along with increased oxygen consumption per meter walked.30
Selecting the appropriate amputation level is vital to optimize functional outcomes without compromising wound healing. Successful prosthetic limb fitting depends largely on the length of the residual stump to support the body load and suspend the prosthesis. For long bone amputations, maintaining at least 12-cm clearance above the knee joint in transfemoral amputees and 10-cm below the knee joint in transtibial amputees is critical for maximizing functional outcomes.31
Surgical technique also is paramount. The goal is to minimize the risk for pressure ulcers by avoiding bony spurs and muscle imbalances. Shaping the muscle and residual limb is essential for proper prosthesis fitting. Attention to neurovascular structures, such as burying nerve ends to prevent neuropathic pain during prosthesis wear, is crucial.32 In extremity amputations, surgeons often resort to free flap transfer techniques for stump reconstruction. In a study of 31 patients with severe lower extremity injuries undergoing various amputations, the use of latissimus dorsi myocutaneous flaps, alone or in combination with serratus anterior muscle flaps, resulted in fewer instances of deep ulceration and allowed for earlier prosthesis wear.33
Addressing Barriers to Wound Healing
Multiple barriers to successful wound healing are encountered in the amputee population. Amputations from trauma have a less-controlled initiation, which carries with it a higher risk for infection, poor wound healing, and other complications.
Infection—Infection often is one of the first hurdles encountered in postamputation wound healing. Critical first steps in infection prevention include thorough cleaning of soiled traumatic wounds and appropriate tissue debridement coupled with scrupulous sterile technique and postoperative monitoring for signs and symptoms of infection.
In a retrospective study of 223 combat-related major lower extremity amputations (initial and revision) between 2009 and 2015, the use of intrawound antibiotic powder at the time of closure demonstrated a 13% absolute risk reduction in deep infection rates, which was particularly notable in revision amputations, with a number needed to treat of 8 for initial amputations and 4 for revision amputations on previously infected limbs.34 Intra-operative antibiotic powder may represent a cheap and easy consideration for this special population of amputees. Postamputation antibiotic prophylaxis for infection prevention is an area of controversy. For nontraumatic infections, data suggest antibiotic prophylaxis may not decrease infection rates in these patients.35,36
Interestingly, a study by Azarbal et al37 aimed to investigate the correlation between nasal methicillin-resistant Staphylococcus aureus (MRSA) colonization and other patient factors with wound occurrence following major lower extremity amputation. The study found MRSA colonization was associated with higher rates of overall wound occurrence as well as wound occurrence due to wound infection. These data suggest nasal MRSA eradication may improve postoperative wound outcomes after major lower extremity amputation.37
Dressing Choice—The dressing chosen for a residual limb also is of paramount importance following amputation. The personalized and dynamic management of postamputation wounds and skin involves achieving optimal healing through a dressing that sustains appropriate moisture levels, addresses edema, helps prevent contractures, and safeguards the limb.38 From the start, using negative pressure wound dressings after surgical amputation can decrease wound-related complications.39
Topical oxygen therapy following amputation also shows promise. In a retrospective case series by Kalliainen et al,40 topical oxygen therapy applied to 58 wounds in 32 patients over 9 months demonstrated positive outcomes in promoting wound healing, with 38 wounds (66%) healing completely with the use of topical oxygen. Minimal complications and no detrimental effects were observed.40
Current recommendations suggest that non–weight-bearing removable rigid dressings are the superior postoperative management for transtibial amputations compared to soft dressings, offering benefits such as faster healing, reduced limb edema, earlier ambulation, preparatory shaping for prosthetic use, and prevention of knee flexion contractures.41-46 Similarly, adding a silicone liner following amputation significantly reduced the duration of prosthetic rehabilitation compared with a conventional soft dressing program in one study (P<.05).47
Specifically targeting wound edema, a case series by Hoskins et al48 investigated the impact of prostheses with vacuum-assisted suspension on the size of residual limb wounds in individuals with transtibial amputation. Well-fitting sockets with vacuum-assisted suspension did not impede wound healing, and the results suggest the potential for continued prosthesis use during the healing process.48 However, a study by Johannesson et al49 compared the outcomes of transtibial amputation patients using a vacuum-formed rigid dressing and a conventional rigid plaster dressing, finding no significant differences in wound healing, time to prosthetic fitting, or functional outcomes with the prosthesis between the 2 groups. When comparing elastic bandaging, pneumatic prosthesis, and temporary prosthesis on postoperative stump management, temporary prosthesis led to a decrease in stump volume, quicker transition to a permanent prosthesis, and improved quality of life compared with elastic bandaging and pneumatic prosthetics.50
The type of material in dressings may contribute to utility in amputation wounds. Keratin-based wound dressings show promise for wound healing, especially in recalcitrant vascular wounds.51 There also are numerous proprietary wound dressings available for patients, at least one of which has particularly thorough data. In a retrospective study of more than 2 million lower extremity wounds across 644 institutions, a proprietary bioactive human skin allograft (TheraSkin [LifeNet Health]) demonstrated higher healing rates, greater percentage area reductions, lower amputations, reduced recidivism, higher treatment completion, and fewer medical transfers compared with standard of care alone.52
Postamputation Dermatologic Concerns
After the postamputation wound heals, a notable concern is the prevalence of skin diseases affecting residual limbs. The stump site in amputees, marked by a delicate cutaneous landscape vulnerable to skin diseases, faces challenges arising from amputation-induced damage to various structures.53
When integrated into a prosthesis socket, the altered skin must acclimate to a humid environment and endure forces for which it is not well suited, especially during movement.53 Amputation remarkably alters normal tissue perfusion, which can lead to aberrant blood and lymphatic circulation in residual limbs.27,53 This compromised skin, often associated with a history of vascular disease, diabetes mellitus, or malignancy, becomes immunocompromised, heightening the risk for dermatologic issues such as inflammation, infection, and malignancies.53 Unlike the resilient volar skin on palms and soles, stump skin lacks adaptation to withstand the compressive forces generated during ambulation, sometimes leading to skin disease and pain that result in abandonment of the prosthesis.53,54 Mechanical forces on the skin, especially in active patients eager to resume pre-injury lifestyles, contribute to skin breakdown. The dynamic nature of the residual limb, including muscle atrophy, gait changes, and weight fluctuations, complicates the prosthetic fitting process. Prosthesis abandonment remains a challenge, despite modern technologic advancements.
The occurrence of heterotopic ossification (extraskeletal bone formation) is another notable issue in military amputees.27,55-57 Poor prosthetic fit can lead to skin degradation, necessitating further surgery to address mispositioned bone formations. Orthopedic monitoring supplemented by appropriate imaging studies can benefit postamputation patients by detecting and preventing heterotopic ossification in its early stages.
Dermatologic issues, especially among lower limb amputees, are noteworthy, with a substantial percentage experiencing complications related to socket prosthetics, such as heat, sweating, sores, and skin irritation. Up to 41% of patients are seen regularly for a secondary skin disorder following amputation.58 As one might expect, persistent wounds, blisters, ulcers, and abscesses are some of the most typical cutaneous abnormalities affecting residual limbs with prostheses.27,58 More rare skin conditions also are documented in residual limbs, including cutaneous granuloma, verrucous carcinoma, bullous pemphigoid, and angiodermatitis.27,59-61
Treatments offered in the dermatology clinic often are similar to patients who have not had an amputation. For instance, hyperhidrosis can be treated with prescription antiperspirant, topical aluminum chloride, topical glycopyrronium, botulinum toxin, and iontophoresis, which can greatly decrease skin irritation and malodor. Subcutaneous neurotoxins such as botulinum toxin are especially useful for hyperhidrosis following amputation because a single treatment can last 3 to 6 months, whereas topicals must be applied multiple times per day and can be inherently irritating to the skin.27,62 Furthermore, ablative fractional resurfacing lasers also can help stimulate new collagen growth, increase skin mobility on residual limbs, smooth jagged scars, and aid prosthetic fitting.27,63 Perforated prosthetic liners also may be useful to address issues such as excessive sweating, demonstrating improvements in skin health, reduced sweating problems, and potential avoidance of surgical interventions.64
When comorbid skin conditions are at bay, preventive measures for excessive wound healing necessitate early recognition and timely intervention for residual limbs. Preventive techniques encompass the use of silicone gel sheeting, hypoallergenic microporous tape, and intralesional steroid injections.
Psychological Concerns—An overarching issue following amputation is the psychological toll the process imposes on the patient. Psychological concerns, including anxiety and depression, present additional challenges impacting residual limb hygiene and prosthetic maintenance. Chronic wounds are devastating to patients. These patients consistently express feeling ostracized from their community and anxious about unemployment, leaking fluid, or odor from the wound, as well as other social stigmata.62 Depression and anxiety can hinder a patient’s ability to care for their wound and make them more susceptible to the myriad issues that can ensue.
Recent Developments in Wound Healing
Wound healing is ripe for innovation that could assuage ailments that impact patients following amputation. A 2022 study by Abu El Hawa et al65 illustrated advanced progression in wound healing for patients taking statins, even though the statin group had increased age and number of comorbidities compared with patients not taking statins.
Nasseri and Sharifi66 showed the potential of antimicrobial peptides—small proteins with cationic charges and amphipathic structures exhibiting electrostatic interaction with microbial cell membranes—in promoting wound healing, particularly defensins and cathelicidin LL-37.They also discussed innovative delivery systems, such as nanoparticles and electrospun fibrous scaffolds, highlighting their potential as possibly more effective therapeutics than antibiotics, especially in the context of diabetic wound closure.66 Aimed at increased angiogenesis in the proliferative phase, there is evidence that N-acetylcysteine can increase amputation stump perfusion with the goal of better long-term wound healing and more efficient scar formation.67
Stem cell therapy, particularly employing cells from the human amniotic membrane, represents an auspicious avenue for antifibrotic treatment. Amniotic epithelial cells and amniotic mesenchymal cells, with their self-renewal and multilineage differentiation capabilities, exhibit anti-inflammatory and antifibrotic properties.4,5 A study by Dong et al68 aimed to assess the efficacy of cell therapy, particularly differentiated progenitor cell–based graft transplantation or autologous stem cell injection, in treating refractory skin injuries such as nonrevascularizable critical limb ischemic ulcers, venous leg ulcers, and diabetic lower limb ulcers. The findings demonstrated cell therapy effectively reduced the size of ulcers, improved wound closure rates, and decreased major amputation rates compared with standard therapy. Of note, cell therapy had limited impact on alleviating pain in patients with critical limb ischemia-related cutaneous ulcers.68
Final Thoughts
Wound care following amputation is a multidisciplinary endeavor, necessitating collaboration between many health care professionals. Dermatologists play a crucial role in providing routine care as well as addressing wound healing and related skin issues among amputation patients. As the field progresses, dermatologists are well positioned to make notable contributions and ensure enhanced outcomes, resulting in a better quality of life for patients facing the challenges of limb amputation and prosthetic use.
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525-549. doi:10.1146/annurev.cellbio.24.110707.175336
- Eming SA, Hammerschmidt M, Krieg T, et al. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20:517-527. doi:10.1016/j.semcdb.2009.04.009
- Eming SA. Evolution of immune pathways in regeneration and repair: recent concepts and translational perspectives. Semin Immunol. 2014;26:275-276. doi:10.1016/j.smim.2014.09.001
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 4th edition. Elsevier; 2018.
- Wang PH, Huang BS, Horng HC, et al. Wound healing. J Chin Med Assoc JCMA. 2018;81:94-101. doi:10.1016/j.jcma.2017.11.002
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528-1542. doi:10.1177/147323000903700531
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321. doi:10.1038/nature07039
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi:10.1126/scitranslmed.3009337
- Eming SA, Brachvogel B, Odorisio T, et al. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115-170. doi:10.1016/j.proghi.2007.06.001
- Janis JE, Harrison B. Wound healing: part I. basic science. Plast Reconstr Surg. 2016;138(3 suppl):9S-17S. doi:10.1097/PRS.0000000000002773
- Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics. part I: the molecular basis of scar formation. J Am Acad Dermatol. 2012;66:1-10; quiz 11-12. doi:10.1016/j.jaad.2011.05.055
- Kwan P, Ding J, Tredget EE. MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS One. 2015;10:e0123054. doi:10.1371/journal.pone.0123054
- Ben W, Yang Y, Yuan J, et al. Human papillomavirus 16 E6 modulates the expression of host microRNAs in cervical cancer. Taiwan J Obstet Gynecol. 2015;54:364-370. doi:10.1016/j.tjog.2014.06.007
- Yu EH, Tu HF, Wu CH, et al. MicroRNA-21 promotes perineural invasion and impacts survival in patients with oral carcinoma. J Chin Med Assoc JCMA. 2017;80:383-388. doi:10.1016/j.jcma.2017.01.003
- Wen KC, Sung PL, Yen MS, et al. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J Obstet Gynecol. 2013;52:465-469. doi:10.1016/j.tjog.2013.10.002
- Babalola O, Mamalis A, Lev-Tov H, et al. The role of microRNAs in skin fibrosis. Arch Dermatol Res. 2013;305:763-776. doi:10.1007/s00403-013-1410-1
- Hofer M, Hoferová Z, Falk M. Pharmacological modulation of radiation damage. does it exist a chance for other substances than hematopoietic growth factors and cytokines? Int J Mol Sci. 2017;18:1385. doi:10.3390/ijms18071385
- Darby IA, Weller CD. Aspirin treatment for chronic wounds: potential beneficial and inhibitory effects. Wound Repair Regen. 2017;25:7-12. doi:10.1111/wrr.12502
- Khalid KA, Nawi AFM, Zulkifli N, et al. Aging and wound healing of the skin: a review of clinical and pathophysiological hallmarks. Life. 2022;12:2142. doi:10.3390/life12122142
- Peacock HM, Gilbert EAB, Vickaryous MK. Scar‐free cutaneous wound healing in the leopard gecko, Eublepharis macularius. J Anat. 2015;227:596-610. doi:10.1111/joa.12368
- Delorme SL, Lungu IM, Vickaryous MK. Scar‐free wound healing and regeneration following tail loss in the leopard gecko, Eublepharis macularius. Anat Rec. 2012;295:1575-1595. doi:10.1002/ar.22490
- Brunauer R, Xia IG, Asrar SN, et al. Aging delays epimorphic regeneration in mice. J Gerontol Ser A Biol Sci Med Sci. 2021;76:1726-1733. doi:10.1093/gerona/glab131
- Dolan CP, Yang TJ, Zimmel K, et al. Epimorphic regeneration of the mouse digit tip is finite. Stem Cell Res Ther. 2022;13:62. doi:10.1186/s13287-022-02741-2
- Simkin J, Han M, Yu L, et al. The mouse digit tip: from wound healing to regeneration. Methods Mol Biol Clifton NJ. 2013;1037:419-435. doi:10.1007/978-1-62703-505-7_24
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429. doi:10.1016/j.apmr.2007.11.005
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663. doi:10.1016/j.apmr.2004.09.003
- Lannan FM, Meyerle JH. The dermatologist’s role in amputee skin care. Cutis. 2019;103:86-90.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777. doi:10.1016/j.injury.2009.02.014
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385. doi:10.1682/jrrd.2009.03.0023
- Pinzur MS, Gold J, Schwartz D, et al. Energy demands for walking in dysvascular amputees as related to the level of amputation. Orthopedics. 1992;15:1033-1036; discussion 1036-1037. doi:10.3928/0147-7447-19920901-07
- Robinson V, Sansam K, Hirst L, et al. Major lower limb amputation–what, why and how to achieve the best results. Orthop Trauma. 2010;24:276-285. doi:10.1016/j.mporth.2010.03.017
- Lu S, Wang C, Zhong W, et al. Amputation stump revision using a free sural neurocutaneous perforator flap. Ann Plast Surg. 2016;76:83-87. doi:10.1097/SAP.0000000000000211
- Kim SW, Jeon SB, Hwang KT, et al. Coverage of amputation stumps using a latissimus dorsi flap with a serratus anterior muscle flap: a comparative study. Ann Plast Surg. 2016;76:88-93. doi:10.1097/SAP.0000000000000220
- Pavey GJ, Formby PM, Hoyt BW, et al. Intrawound antibiotic powder decreases frequency of deep infection and severity of heterotopic ossification in combat lower extremity amputations. Clin Orthop. 2019;477:802-810. doi:10.1007/s11999.0000000000000090
- Dunkel N, Belaieff W, Assal M, et al. Wound dehiscence and stump infection after lower limb amputation: risk factors and association with antibiotic use. J Orthop Sci Off J Jpn Orthop Assoc. 2012;17:588-594. doi:10.1007/s00776-012-0245-5
- Rubin G, Orbach H, Rinott M, et al. The use of prophylactic antibiotics in treatment of fingertip amputation: a randomized prospective trial. Am J Emerg Med. 2015;33:645-647. doi:10.1016/j.ajem.2015.02.002
- Azarbal AF, Harris S, Mitchell EL, et al. Nasal methicillin-resistant Staphylococcus aureus colonization is associated with increased wound occurrence after major lower extremity amputation. J Vasc Surg. 2015;62:401-405. doi:10.1016/j.jvs.2015.02.052
- Kwasniewski M, Mitchel D. Post amputation skin and wound care. Phys Med Rehabil Clin N Am. 2022;33:857-870. doi:10.1016/j.pmr.2022.06.010
- Chang H, Maldonado TS, Rockman CB, et al. Closed incision negative pressure wound therapy may decrease wound complications in major lower extremity amputations. J Vasc Surg. 2021;73:1041-1047. doi:10.1016/j.jvs.2020.07.061
- Kalliainen LK, Gordillo GM, Schlanger R, et al. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiol Off J Int Soc Pathophysiol. 2003;9:81-87. doi:10.1016/s0928-4680(02)00079-2
- Reichmann JP, Stevens PM, Rheinstein J, et al. Removable rigid dressings for postoperative management of transtibial amputations: a review of published evidence. PM R. 2018;10:516-523. doi:10.1016/j.pmrj.2017.10.002
- MacLean N, Fick GH. The effect of semirigid dressings on below-knee amputations. Phys Ther. 1994;74:668-673. doi:10.1093/ptj/74.7.668
- Koonalinthip N, Sukthongsa A, Janchai S. Comparison of removable rigid dressing and elastic bandage for residual limb maturation in transtibial amputees: a randomized controlled trial. Arch Phys Med Rehabil. 2020;101:1683-1688. doi:10.1016/j.apmr.2020.05.009
- Taylor L, Cavenett S, Stepien JM, et al. Removable rigid dressings: a retrospective case-note audit to determine the validity of post-amputation application. Prosthet Orthot Int. 2008;32:223-230. doi:10.1080/03093640802016795
- Sumpio B, Shine SR, Mahler D, et al. A comparison of immediate postoperative rigid and soft dressings for below-knee amputations. Ann Vasc Surg. 2013;27:774-780. doi:10.1016/j.avsg.2013.03.007
- van Velzen AD, Nederhand MJ, Emmelot CH, et al. Early treatment of trans-tibial amputees: retrospective analysis of early fitting and elastic bandaging. Prosthet Orthot Int. 2005;29:3-12. doi:10.1080/17461550500069588
- Chin T, Toda M. Results of prosthetic rehabilitation on managing transtibial vascular amputation with silicone liner after wound closure. J Int Med Res. 2016;44:957-967. doi:10.1177/0300060516647554
- Hoskins RD, Sutton EE, Kinor D, et al. Using vacuum-assisted suspension to manage residual limb wounds in persons with transtibial amputation: a case series. Prosthet Orthot Int. 2014;38:68-74. doi:10.1177/0309364613487547
- Johannesson A, Larsson GU, Oberg T, et al. Comparison of vacuum-formed removable rigid dressing with conventional rigid dressing after transtibial amputation: similar outcome in a randomized controlled trial involving 27 patients. Acta Orthop. 2008;79:361-369. doi:10.1080/17453670710015265
- Alsancak S, Köse SK, Altınkaynak H. Effect of elastic bandaging and prosthesis on the decrease in stump volume. Acta Orthop Traumatol Turc. 2011;45:14-22. doi:10.3944/AOTT.2011.2365
- Than MP, Smith RA, Hammond C, et al. Keratin-based wound care products for treatment of resistant vascular wounds. J Clin Aesthetic Dermatol. 2012;5:31-35.
- Gurtner GC, Garcia AD, Bakewell K, et al. A retrospective matched‐cohort study of 3994 lower extremity wounds of multiple etiologies across 644 institutions comparing a bioactive human skin allograft, TheraSkin, plus standard of care, to standard of care alone. Int Wound J. 2020;17:55-64. doi:10.1111/iwj.13231
- Buikema KES, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677. doi:10.1016/j.clindermatol.2014.04.015
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286. doi:10.1001/archdermatol.2012.3004
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486. doi:10.2106/JBJS.F.00412
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30. doi:10.1097/BOT.0000000000000666
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237. doi:10.1097/BOT.0b013e3182a53130
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump. Clin Exp Dermatol. 2011;36:560-561. doi:10.1111/j.1365-2230.2011.04037.x
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb. Int J Dermatol. 2012;51:75-78. doi:10.1111/j.1365-4632.2011.04906.x
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatologica. 1991;182:193-195. doi:10.1159/000247782
- Campanati A, Diotallevi F, Radi G, et al. Efficacy and safety of botulinum toxin B in focal hyperhidrosis: a narrative review. Toxins. 2023;15:147. doi:10.3390/toxins15020147
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193. doi:10.1001/jamadermatol.2013.7761
- McGrath M, McCarthy J, Gallego A, et al. The influence of perforated prosthetic liners on residual limb wound healing: a case report. Can Prosthet Orthot J. 2019;2:32723. doi:10.33137/cpoj.v2i1.32723
- Abu El Hawa AA, Klein D, Bekeny JC, et al. The impact of statins on wound healing: an ally in treating the highly comorbid patient. J Wound Care. 2022;31(suppl 2):S36-S41. doi:10.12968/jowc.2022.31.Sup2.S36
- Nasseri S, Sharifi M. Therapeutic potential of antimicrobial peptides for wound healing. Int J Pept Res Ther. 2022;28:38. doi:10.1007/s10989-021-10350-5
- Lee JV, Engel C, Tay S, et al. N-Acetyl-Cysteine treatment after lower extremity amputation improves areas of perfusion defect and wound healing outcomes. J Vasc Surg. 2021;73:39-40. doi:10.1016/j.jvs.2020.12.025
- Dong Y, Yang Q, Sun X. Comprehensive analysis of cell therapy on chronic skin wound healing: a meta-analysis. Hum Gene Ther. 2021;32:787-795. doi:10.1089/hum.2020.275
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525-549. doi:10.1146/annurev.cellbio.24.110707.175336
- Eming SA, Hammerschmidt M, Krieg T, et al. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20:517-527. doi:10.1016/j.semcdb.2009.04.009
- Eming SA. Evolution of immune pathways in regeneration and repair: recent concepts and translational perspectives. Semin Immunol. 2014;26:275-276. doi:10.1016/j.smim.2014.09.001
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 4th edition. Elsevier; 2018.
- Wang PH, Huang BS, Horng HC, et al. Wound healing. J Chin Med Assoc JCMA. 2018;81:94-101. doi:10.1016/j.jcma.2017.11.002
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528-1542. doi:10.1177/147323000903700531
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321. doi:10.1038/nature07039
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi:10.1126/scitranslmed.3009337
- Eming SA, Brachvogel B, Odorisio T, et al. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115-170. doi:10.1016/j.proghi.2007.06.001
- Janis JE, Harrison B. Wound healing: part I. basic science. Plast Reconstr Surg. 2016;138(3 suppl):9S-17S. doi:10.1097/PRS.0000000000002773
- Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics. part I: the molecular basis of scar formation. J Am Acad Dermatol. 2012;66:1-10; quiz 11-12. doi:10.1016/j.jaad.2011.05.055
- Kwan P, Ding J, Tredget EE. MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS One. 2015;10:e0123054. doi:10.1371/journal.pone.0123054
- Ben W, Yang Y, Yuan J, et al. Human papillomavirus 16 E6 modulates the expression of host microRNAs in cervical cancer. Taiwan J Obstet Gynecol. 2015;54:364-370. doi:10.1016/j.tjog.2014.06.007
- Yu EH, Tu HF, Wu CH, et al. MicroRNA-21 promotes perineural invasion and impacts survival in patients with oral carcinoma. J Chin Med Assoc JCMA. 2017;80:383-388. doi:10.1016/j.jcma.2017.01.003
- Wen KC, Sung PL, Yen MS, et al. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J Obstet Gynecol. 2013;52:465-469. doi:10.1016/j.tjog.2013.10.002
- Babalola O, Mamalis A, Lev-Tov H, et al. The role of microRNAs in skin fibrosis. Arch Dermatol Res. 2013;305:763-776. doi:10.1007/s00403-013-1410-1
- Hofer M, Hoferová Z, Falk M. Pharmacological modulation of radiation damage. does it exist a chance for other substances than hematopoietic growth factors and cytokines? Int J Mol Sci. 2017;18:1385. doi:10.3390/ijms18071385
- Darby IA, Weller CD. Aspirin treatment for chronic wounds: potential beneficial and inhibitory effects. Wound Repair Regen. 2017;25:7-12. doi:10.1111/wrr.12502
- Khalid KA, Nawi AFM, Zulkifli N, et al. Aging and wound healing of the skin: a review of clinical and pathophysiological hallmarks. Life. 2022;12:2142. doi:10.3390/life12122142
- Peacock HM, Gilbert EAB, Vickaryous MK. Scar‐free cutaneous wound healing in the leopard gecko, Eublepharis macularius. J Anat. 2015;227:596-610. doi:10.1111/joa.12368
- Delorme SL, Lungu IM, Vickaryous MK. Scar‐free wound healing and regeneration following tail loss in the leopard gecko, Eublepharis macularius. Anat Rec. 2012;295:1575-1595. doi:10.1002/ar.22490
- Brunauer R, Xia IG, Asrar SN, et al. Aging delays epimorphic regeneration in mice. J Gerontol Ser A Biol Sci Med Sci. 2021;76:1726-1733. doi:10.1093/gerona/glab131
- Dolan CP, Yang TJ, Zimmel K, et al. Epimorphic regeneration of the mouse digit tip is finite. Stem Cell Res Ther. 2022;13:62. doi:10.1186/s13287-022-02741-2
- Simkin J, Han M, Yu L, et al. The mouse digit tip: from wound healing to regeneration. Methods Mol Biol Clifton NJ. 2013;1037:419-435. doi:10.1007/978-1-62703-505-7_24
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429. doi:10.1016/j.apmr.2007.11.005
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663. doi:10.1016/j.apmr.2004.09.003
- Lannan FM, Meyerle JH. The dermatologist’s role in amputee skin care. Cutis. 2019;103:86-90.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777. doi:10.1016/j.injury.2009.02.014
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385. doi:10.1682/jrrd.2009.03.0023
- Pinzur MS, Gold J, Schwartz D, et al. Energy demands for walking in dysvascular amputees as related to the level of amputation. Orthopedics. 1992;15:1033-1036; discussion 1036-1037. doi:10.3928/0147-7447-19920901-07
- Robinson V, Sansam K, Hirst L, et al. Major lower limb amputation–what, why and how to achieve the best results. Orthop Trauma. 2010;24:276-285. doi:10.1016/j.mporth.2010.03.017
- Lu S, Wang C, Zhong W, et al. Amputation stump revision using a free sural neurocutaneous perforator flap. Ann Plast Surg. 2016;76:83-87. doi:10.1097/SAP.0000000000000211
- Kim SW, Jeon SB, Hwang KT, et al. Coverage of amputation stumps using a latissimus dorsi flap with a serratus anterior muscle flap: a comparative study. Ann Plast Surg. 2016;76:88-93. doi:10.1097/SAP.0000000000000220
- Pavey GJ, Formby PM, Hoyt BW, et al. Intrawound antibiotic powder decreases frequency of deep infection and severity of heterotopic ossification in combat lower extremity amputations. Clin Orthop. 2019;477:802-810. doi:10.1007/s11999.0000000000000090
- Dunkel N, Belaieff W, Assal M, et al. Wound dehiscence and stump infection after lower limb amputation: risk factors and association with antibiotic use. J Orthop Sci Off J Jpn Orthop Assoc. 2012;17:588-594. doi:10.1007/s00776-012-0245-5
- Rubin G, Orbach H, Rinott M, et al. The use of prophylactic antibiotics in treatment of fingertip amputation: a randomized prospective trial. Am J Emerg Med. 2015;33:645-647. doi:10.1016/j.ajem.2015.02.002
- Azarbal AF, Harris S, Mitchell EL, et al. Nasal methicillin-resistant Staphylococcus aureus colonization is associated with increased wound occurrence after major lower extremity amputation. J Vasc Surg. 2015;62:401-405. doi:10.1016/j.jvs.2015.02.052
- Kwasniewski M, Mitchel D. Post amputation skin and wound care. Phys Med Rehabil Clin N Am. 2022;33:857-870. doi:10.1016/j.pmr.2022.06.010
- Chang H, Maldonado TS, Rockman CB, et al. Closed incision negative pressure wound therapy may decrease wound complications in major lower extremity amputations. J Vasc Surg. 2021;73:1041-1047. doi:10.1016/j.jvs.2020.07.061
- Kalliainen LK, Gordillo GM, Schlanger R, et al. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiol Off J Int Soc Pathophysiol. 2003;9:81-87. doi:10.1016/s0928-4680(02)00079-2
- Reichmann JP, Stevens PM, Rheinstein J, et al. Removable rigid dressings for postoperative management of transtibial amputations: a review of published evidence. PM R. 2018;10:516-523. doi:10.1016/j.pmrj.2017.10.002
- MacLean N, Fick GH. The effect of semirigid dressings on below-knee amputations. Phys Ther. 1994;74:668-673. doi:10.1093/ptj/74.7.668
- Koonalinthip N, Sukthongsa A, Janchai S. Comparison of removable rigid dressing and elastic bandage for residual limb maturation in transtibial amputees: a randomized controlled trial. Arch Phys Med Rehabil. 2020;101:1683-1688. doi:10.1016/j.apmr.2020.05.009
- Taylor L, Cavenett S, Stepien JM, et al. Removable rigid dressings: a retrospective case-note audit to determine the validity of post-amputation application. Prosthet Orthot Int. 2008;32:223-230. doi:10.1080/03093640802016795
- Sumpio B, Shine SR, Mahler D, et al. A comparison of immediate postoperative rigid and soft dressings for below-knee amputations. Ann Vasc Surg. 2013;27:774-780. doi:10.1016/j.avsg.2013.03.007
- van Velzen AD, Nederhand MJ, Emmelot CH, et al. Early treatment of trans-tibial amputees: retrospective analysis of early fitting and elastic bandaging. Prosthet Orthot Int. 2005;29:3-12. doi:10.1080/17461550500069588
- Chin T, Toda M. Results of prosthetic rehabilitation on managing transtibial vascular amputation with silicone liner after wound closure. J Int Med Res. 2016;44:957-967. doi:10.1177/0300060516647554
- Hoskins RD, Sutton EE, Kinor D, et al. Using vacuum-assisted suspension to manage residual limb wounds in persons with transtibial amputation: a case series. Prosthet Orthot Int. 2014;38:68-74. doi:10.1177/0309364613487547
- Johannesson A, Larsson GU, Oberg T, et al. Comparison of vacuum-formed removable rigid dressing with conventional rigid dressing after transtibial amputation: similar outcome in a randomized controlled trial involving 27 patients. Acta Orthop. 2008;79:361-369. doi:10.1080/17453670710015265
- Alsancak S, Köse SK, Altınkaynak H. Effect of elastic bandaging and prosthesis on the decrease in stump volume. Acta Orthop Traumatol Turc. 2011;45:14-22. doi:10.3944/AOTT.2011.2365
- Than MP, Smith RA, Hammond C, et al. Keratin-based wound care products for treatment of resistant vascular wounds. J Clin Aesthetic Dermatol. 2012;5:31-35.
- Gurtner GC, Garcia AD, Bakewell K, et al. A retrospective matched‐cohort study of 3994 lower extremity wounds of multiple etiologies across 644 institutions comparing a bioactive human skin allograft, TheraSkin, plus standard of care, to standard of care alone. Int Wound J. 2020;17:55-64. doi:10.1111/iwj.13231
- Buikema KES, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677. doi:10.1016/j.clindermatol.2014.04.015
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286. doi:10.1001/archdermatol.2012.3004
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486. doi:10.2106/JBJS.F.00412
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30. doi:10.1097/BOT.0000000000000666
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237. doi:10.1097/BOT.0b013e3182a53130
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump. Clin Exp Dermatol. 2011;36:560-561. doi:10.1111/j.1365-2230.2011.04037.x
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb. Int J Dermatol. 2012;51:75-78. doi:10.1111/j.1365-4632.2011.04906.x
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatologica. 1991;182:193-195. doi:10.1159/000247782
- Campanati A, Diotallevi F, Radi G, et al. Efficacy and safety of botulinum toxin B in focal hyperhidrosis: a narrative review. Toxins. 2023;15:147. doi:10.3390/toxins15020147
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193. doi:10.1001/jamadermatol.2013.7761
- McGrath M, McCarthy J, Gallego A, et al. The influence of perforated prosthetic liners on residual limb wound healing: a case report. Can Prosthet Orthot J. 2019;2:32723. doi:10.33137/cpoj.v2i1.32723
- Abu El Hawa AA, Klein D, Bekeny JC, et al. The impact of statins on wound healing: an ally in treating the highly comorbid patient. J Wound Care. 2022;31(suppl 2):S36-S41. doi:10.12968/jowc.2022.31.Sup2.S36
- Nasseri S, Sharifi M. Therapeutic potential of antimicrobial peptides for wound healing. Int J Pept Res Ther. 2022;28:38. doi:10.1007/s10989-021-10350-5
- Lee JV, Engel C, Tay S, et al. N-Acetyl-Cysteine treatment after lower extremity amputation improves areas of perfusion defect and wound healing outcomes. J Vasc Surg. 2021;73:39-40. doi:10.1016/j.jvs.2020.12.025
- Dong Y, Yang Q, Sun X. Comprehensive analysis of cell therapy on chronic skin wound healing: a meta-analysis. Hum Gene Ther. 2021;32:787-795. doi:10.1089/hum.2020.275
Practice Points
- Wound healing in adults is a complex dynamic process that usually takes the greater part of 1 year to completely resolve and is marked by the end of scar formation.
- Postamputation residual limbs are subject to mechanical and biophysical stress to which the overlying skin is not accustomed. Skin treatment aims at mitigating these stresses.
- The major dermatologic barriers to successful wound healing following amputation include infection, skin breakdown, formation of chronic wounds and granulation tissue, heterotopic ossification, and hyperhidrosis.
Treatment and Current Policies on Pseudofolliculitis Barbae in the US Military
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.
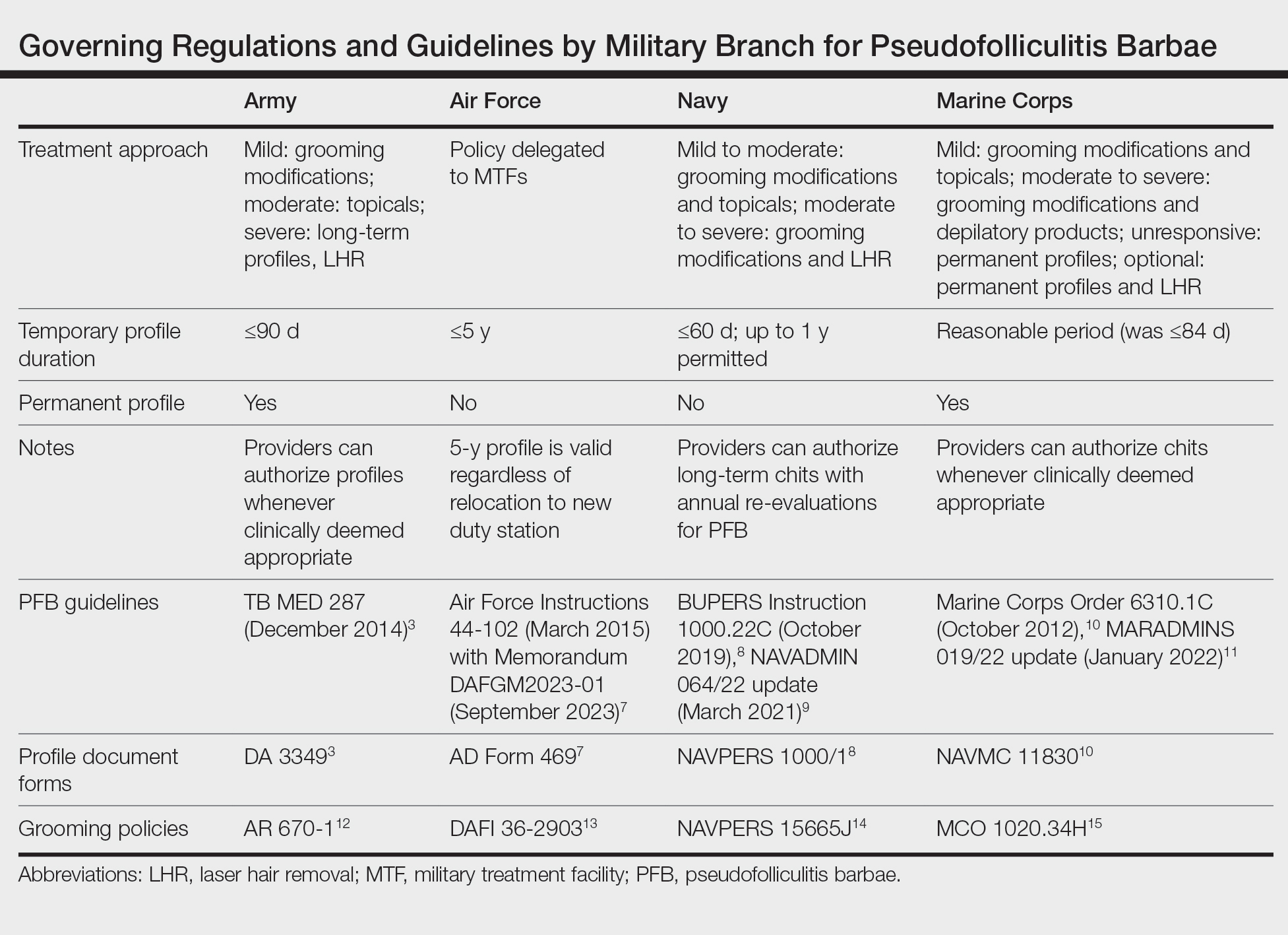
Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Practice Points
- Pseudofolliculitis barbae (PFB) is common among US service members due to grooming standards in the military.
- Each military branch follows separate yet related guidelines to treat PFB.
- The best treatment for severe or refractory cases of PFB is a long-term shaving restriction or laser hair removal.
Sarcoidosis in Post–9/11 Military Veterans
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.
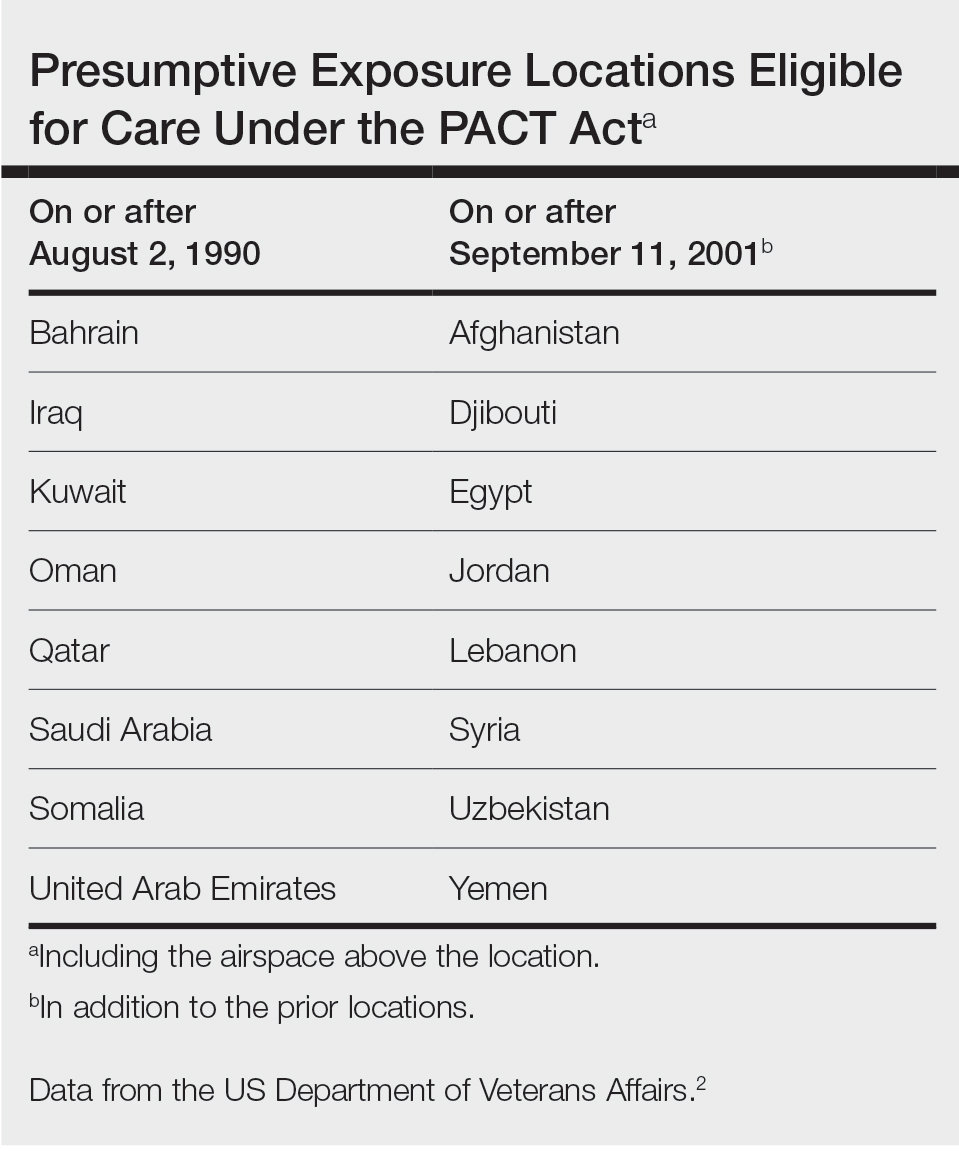
Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18
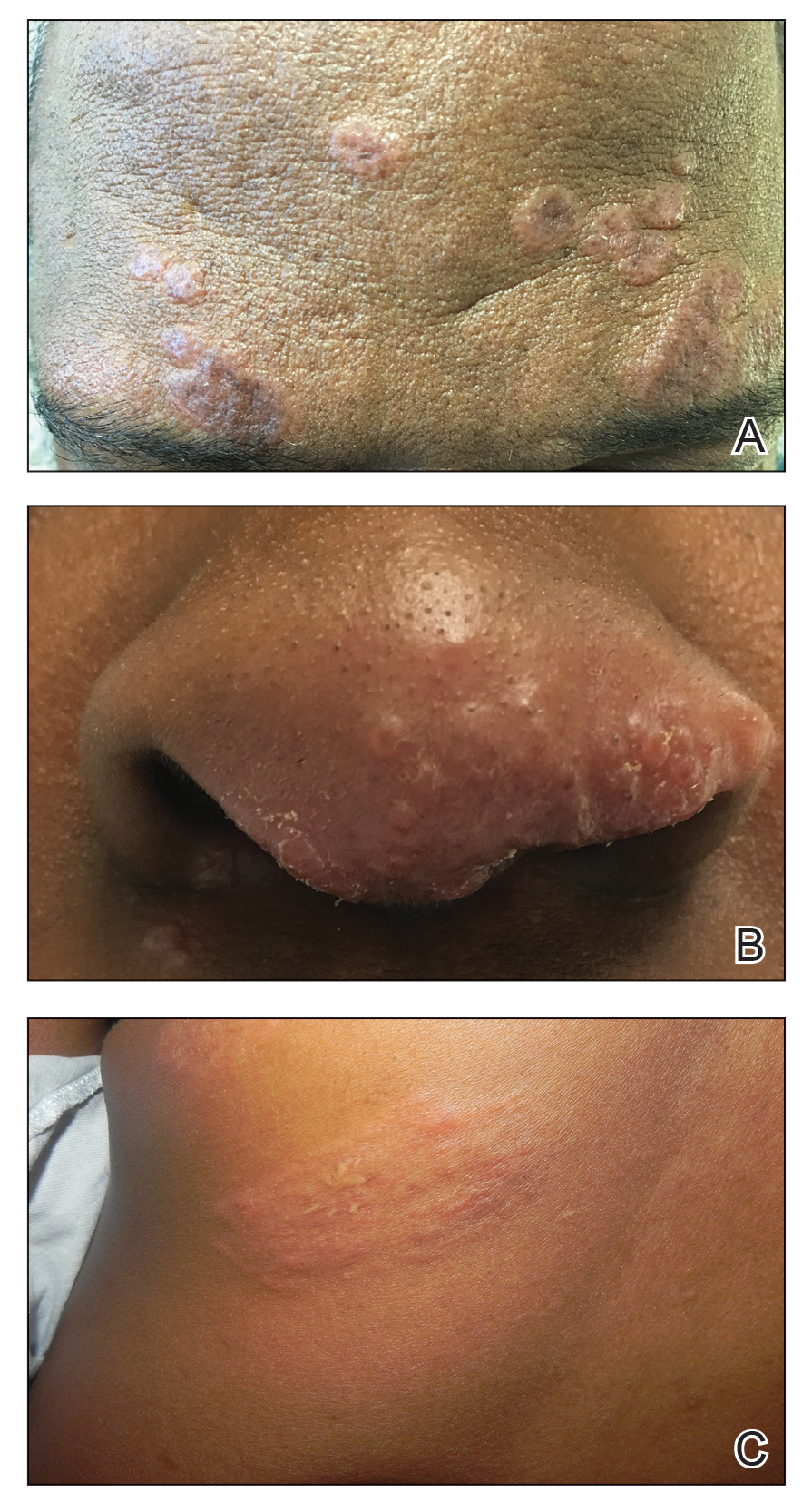
When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).
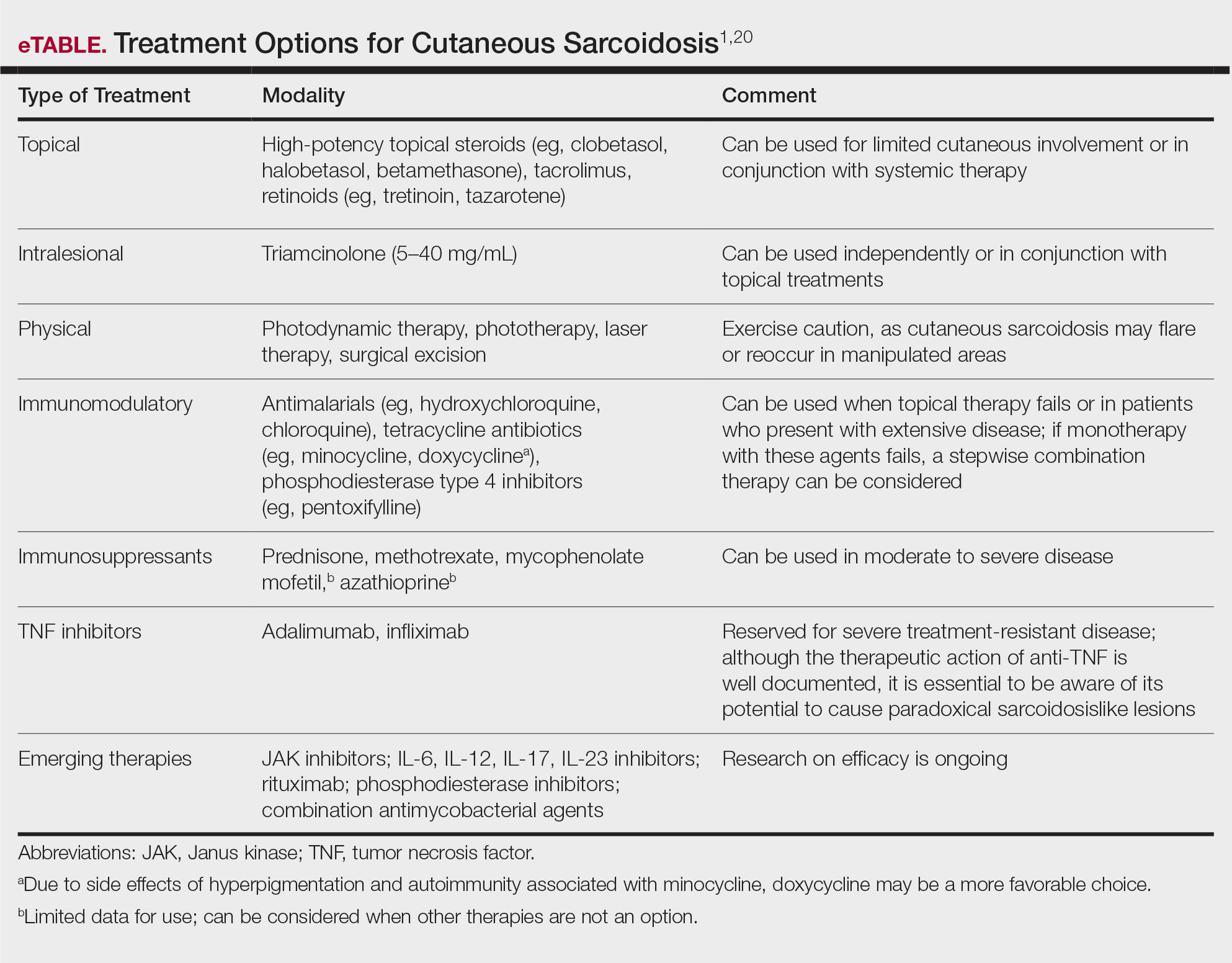
An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.

Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18

When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).

An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.

Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18

When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).

An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
Practice Points
- Cutaneous sarcoidosis is the most common extrapulmonary manifestation of the disease.
- Cutaneous sarcoidosis can precede systemic manifestations of the disease and should prompt further workup.
- Sarcoidosis is a presumptive diagnosis under the PACT Act and may be a service-connected condition. Veterans with presumptive exposures should be referred to the US Department of Veterans Affairs.
Understanding Medical Standards for Entrance Into Military Service and Disqualifying Dermatologic Conditions
Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
Practice Points
- Dermatologic diseases have played a substantial role in conflicts throughout US military history, representing a considerable source of morbidity to service members, loss of active-duty service members trained with necessary skills, and costly use of resources.
- The strict standards are designed to protect the health of the individual and maximize mission success.
- The Department of Defense has a publicly available document (DoD Instruction 6130.03, Volume 1) that details conditions that are disqualifying for entrance into the military. Dermatologists can reference this to provide guidance to adolescents and young adults interested in joining the military.
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Janus Kinase Inhibitors in the Treatment of Atopic Dermatitis: Military Considerations
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
Practice Points
- Oral Janus kinase (JAK) inhibitors are novel therapies available for the treatment of atopic dermatitis (AD), with multiple recently approved agents within the class.
- Recommended laboratory monitoring during treatment with oral JAK inhibitors may limit the use of these medications in the active-duty military population or in those with special-duty assignments.
- The oral and topical bioavailability of these medications makes them a more feasible option for deploying service members or for those requiring flexible dosing.
- The rapid improvement in AD seen in multiple trials of oral JAK inhibitors suggests these agents could prove useful in management of acute AD flares, especially in military environments, where injectable agents are either unavailable or unsupported.
Disparities of Cutaneous Malignancies in the US Military
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Practice Points
- Skin cancer is more prevalent among military personnel and veterans, especially those in the US Air Force. Frequent and/or prolonged sun exposure and lack of sun protection may be key factors.
- Future research should compare the prevalence of skin cancer in nonpilot military populations to the general US population; explore racial and ethnic differences by military branch and their influence on skin cancers; analyze each branch’s sun-protective measures, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure; and explore the effects of appropriate military skin cancer intervention and screening programs.
Cutaneous Body Image: How the Mental Health Benefits of Treating Dermatologic Disease Support Military Readiness in Service Members
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
Practice Points
- The term readiness refers to the ability to recruit, train, deploy, and sustain military forces that are ready to “fight tonight” and succeed in combat.
- Maintaining readiness requires a holistic approach, as it is directly affected by physical and mental health outcomes.
- Cutaneous body image (CBI) refers to an individual’s mental perception of the condition of their hair, nails, and skin. Positive CBI is related to increased quality of life, while negative CBI, which often is associated with dermatologic disease, is associated with poorer health outcomes and even self-injury.
- Treatment of dermatologic disease in the context of active-duty military members can positively influence CBI, which may in turn increase service members’ quality of life and overall military readiness.