User login
Oral Isotretinoin for Acne in the US Military: How Accelerated Courses and Teledermatology Can Minimize the Duty-Limiting Impacts of Treatment
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Practice Points
- Acne is a common skin disease with a high prevalence in the active-duty US Military population.
- Oral isotretinoin is a commonly utilized acne medication that can limit the ability for military service members to deploy and is considered disqualifying for some special duty assignments.
- High daily- and cumulative-dose oral isotretinoin therapy as well as teledermatology can minimize the duty-limiting impact of isotretinoin therapy for military service members.
Cutaneous Cold Weather Injuries in the US Military
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
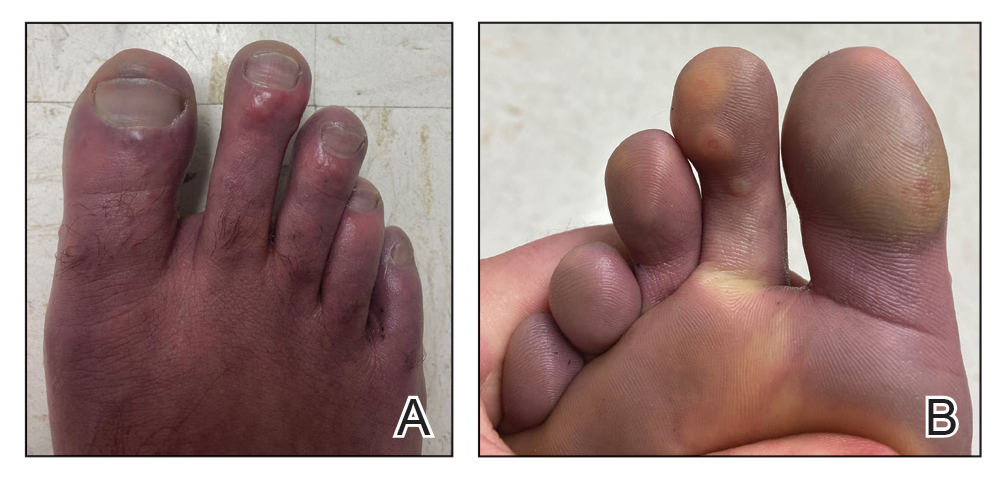
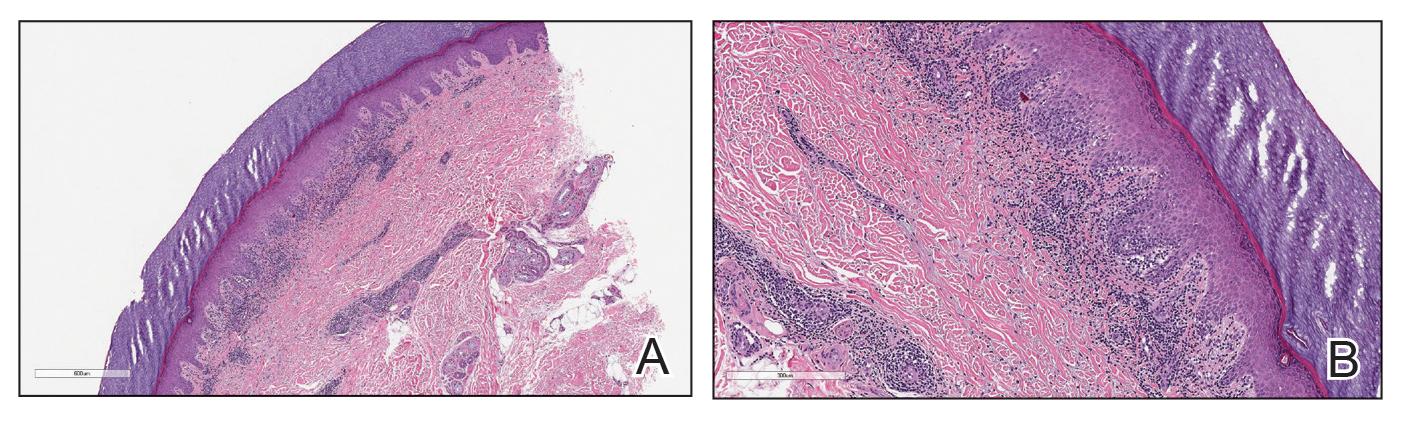
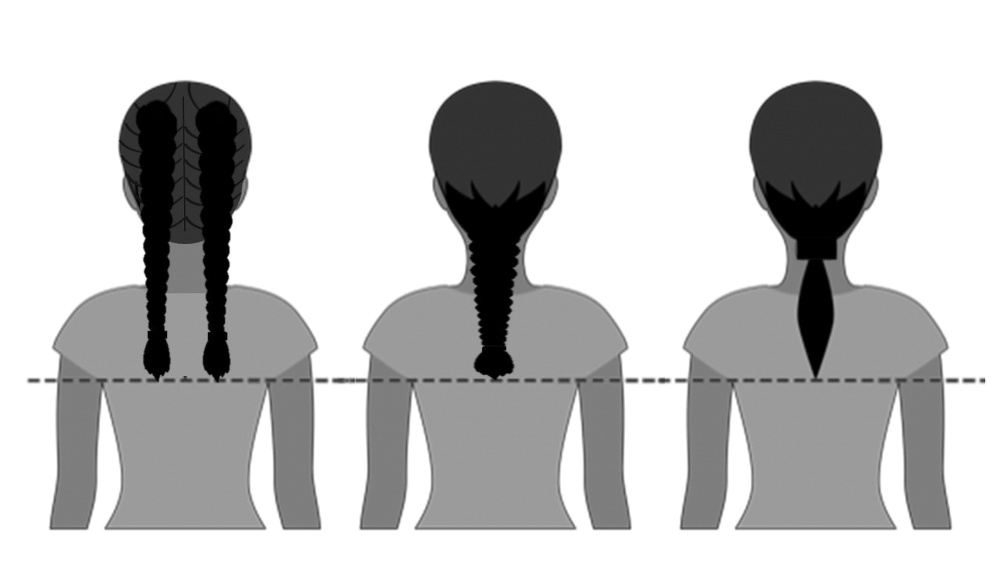
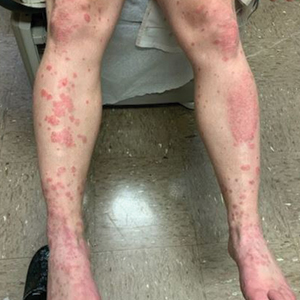
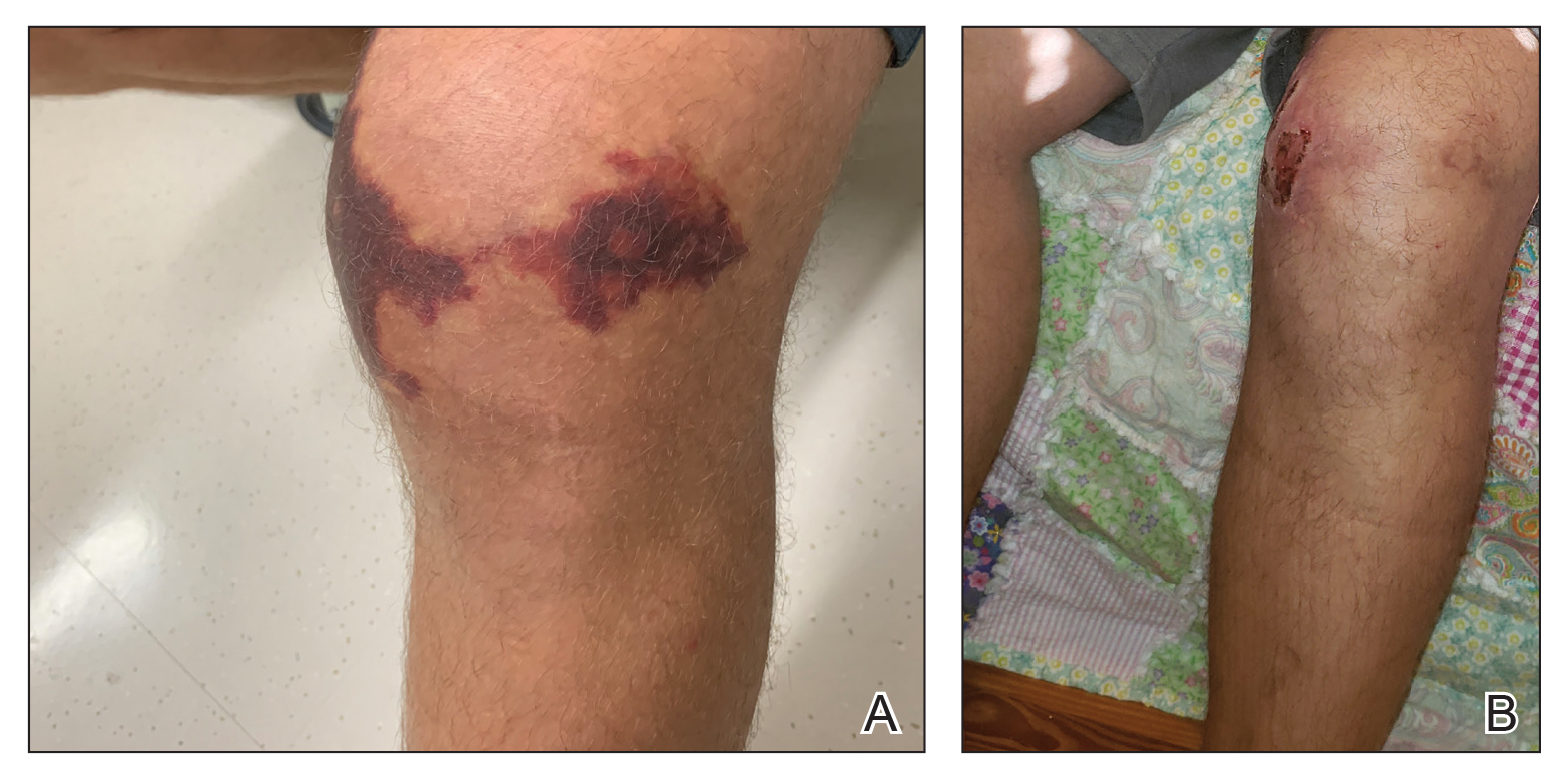
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
Practice Points
- Military service members are at an increased risk for cutaneous cold weather injuries in certain circumstances due to the demands of military training and combat operations.
- Cold weather may cause injury by directly damaging tissues, leading to neurovascular disruption, and by exacerbating existing medical conditions.
From Buns to Braids and Ponytails: Entering a New Era of Female Military Hair-Grooming Standards
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Practice Points
- Military hair-grooming standards have undergone considerable changes to foster inclusivity and acknowledge racial diversity in hair and skin types.
- The chronic wearing of tight hairstyles can lead to hair breakage, headaches, and traction alopecia.
- A deliberate focus on diversity and inclusivity has started to drive policy change that eliminates racial and gender bias.
Apremilast Uses and Relevance to the Military
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Practice Points
- Apremilast is a versatile and easy-to-use therapeutic option for treatment of psoriasis and psoriatic arthritis.
- Ease of transport and storage as well as lack of necessary laboratory monitoring have made apremilast a compelling treatment option for psoriasis and psoriatic arthritis in military populations with high operational tempos.
- Dermatologists should consider apremilast for treatment in populations that work for prolonged periods in austere or resource-limited environments.
Skin Cancer in the US Military
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
Practice Points
- An increased risk for melanoma and keratinocyte carcinomas has been identified in those who have served in the US Military.
- UV radiation exposure, low utilization of sun-protective strategies, and low overall education regarding the risks of UV exposure appear to be the primary contributors to increased risks of skin cancer in this population.
- Improving education for military servicemembers on the risks of UV exposure, increasing utilization of sun-protective clothing, and improving access and utilization of sunscreen are viable options to decrease the risk for cutaneous malignancies in US Military servicemembers.
Hidradenitis Suppurativa in the Military
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
Practice Points
- Hidradenitis suppurativa (HS) can be more difficult to treat in physically active military servicemembers (SMs).
- Patient education and primary care physician awareness of HS is critical to initial diagnosis and long-term management.
- Primary care physician knowledge of HS as well as an understanding of the capabilities at local military medical facilities is important for optimal treatment of HS in military SMs.
Fighting Acne for the Fighting Forces
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.
The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Practice Points
- Acne is a common disease that may cause considerable physical and psychological morbidity. Numerous therapies are available, each with their respective risks and benefits.
- Military servicemembers face unique challenges in the management of acne due to operational and medical readiness considerations.
- Less conventional treatments such as photodynamic therapy and pulsed dye laser may be available to military servicemembers.
- Pulsed dye laser is an effective alternative treatment of acne, especially in an age of growing antibiotic resistance.
Acne Keloidalis Nuchae in the Armed Forces
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Practice Points
- Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder of the occipital scalp and posterior neck characterized by keloidlike papules, pustules, and plaques that develop following mechanical irritation.
- Military members are required to maintain short haircuts and may be disproportionately affected by AKN.
- In the military population, early identification and treatment, which includes topical steroids, oral antibiotics, UV light therapy, lasers, and surgical excision, can prevent further scarring, permanent hair loss, and disfigurement from AKN.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.
Atopic Dermatitis in the US Military
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Practice Points
- The US Military follows strict medical eligibility requirements for enlistment and retention. Atopic dermatitis (AD) and chronic eczematous conditions after 12 years of age is disqualifying for military service, but waivers may be possible for mild cases.
- Unpredictable and rigorous environmental and occupational stressors associated with military service as well as limited access to medical care make AD a challenging condition to manage for service members, particularly during military deployment.
- Accurate diagnosis and documentation of AD in childhood and adolescence by nonmilitary providers are essential, as they will aid in appropriately determining an applicant’s potential to successfully serve in the military.
- For current service members, nonmilitary providers play a vital role in diagnosis and management where military dermatologists are not readily available.