Article
Skin Cancer in the US Military
- Author:
- Ryan Gall, MD
- Michelle Bongiorno, MD
- Kent Handfield, MD, MPH
This review proposes education of individual military servicemembers regarding skin cancer prevention and identification, increasing the...
Article
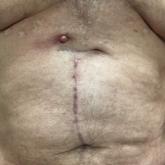
Erythematous Abdominal Nodule
- Author:
- Ryan Gall, MD
- Matthew Willett, MD
- John D. Peters, MD
A 71-year-old man presented with an inflamed erythematous papule on the right subcostal region of 12 months’ duration. It began as a small...
