User login
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Cutaneous Cold Weather Injuries in the US Military
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
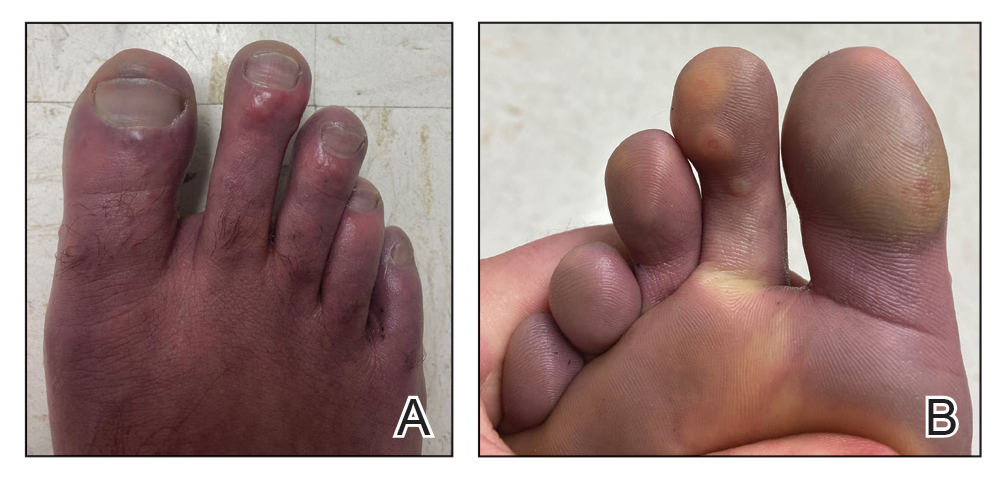
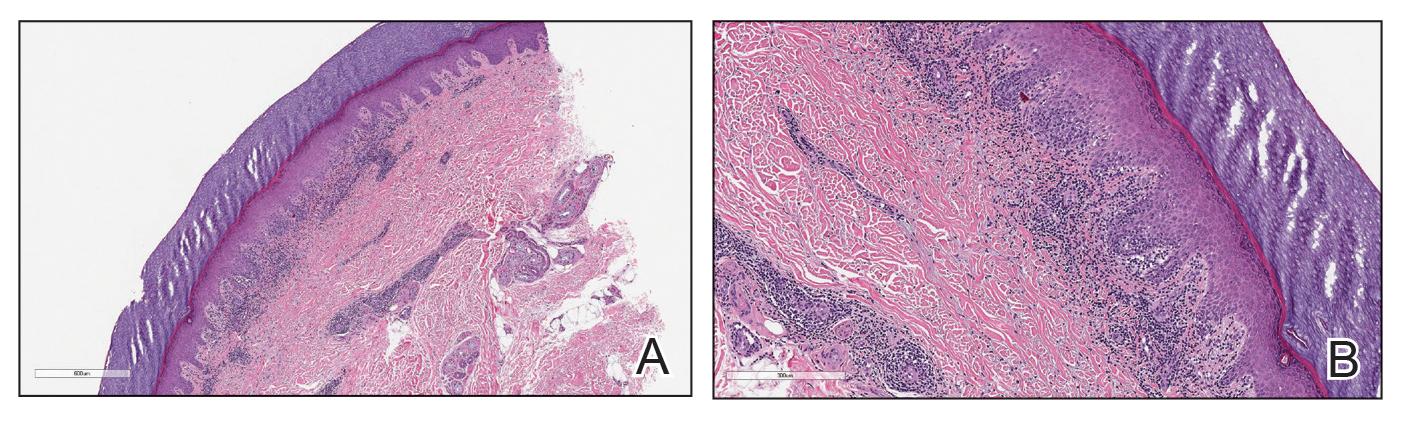
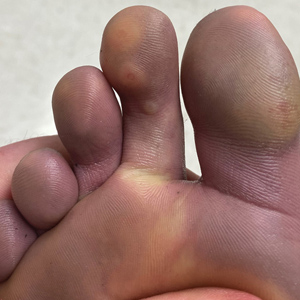
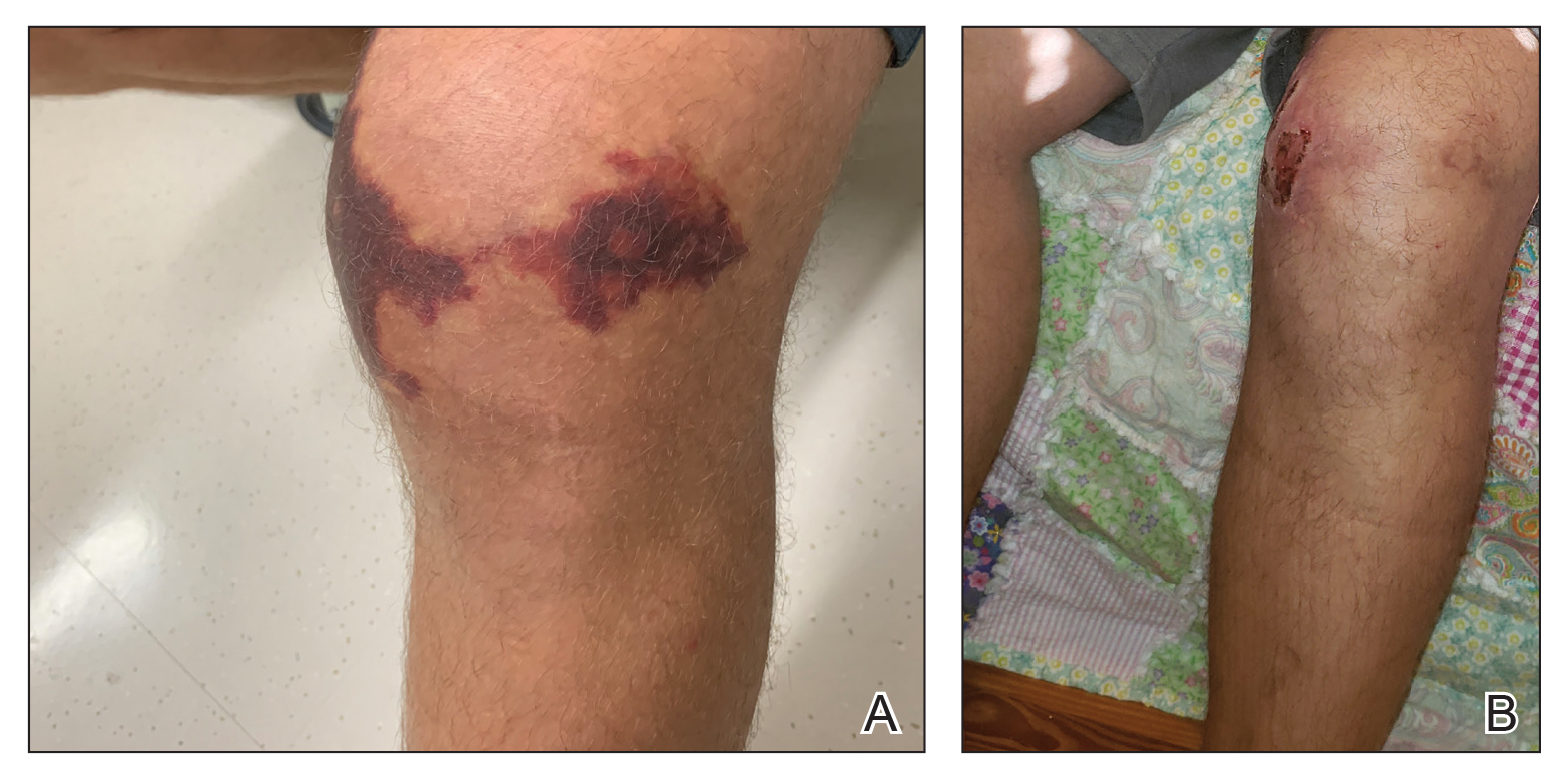
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
Practice Points
- Military service members are at an increased risk for cutaneous cold weather injuries in certain circumstances due to the demands of military training and combat operations.
- Cold weather may cause injury by directly damaging tissues, leading to neurovascular disruption, and by exacerbating existing medical conditions.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.