User login
Disparities of Cutaneous Malignancies in the US Military
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Practice Points
- Skin cancer is more prevalent among military personnel and veterans, especially those in the US Air Force. Frequent and/or prolonged sun exposure and lack of sun protection may be key factors.
- Future research should compare the prevalence of skin cancer in nonpilot military populations to the general US population; explore racial and ethnic differences by military branch and their influence on skin cancers; analyze each branch’s sun-protective measures, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure; and explore the effects of appropriate military skin cancer intervention and screening programs.
Ichthyosiform Sarcoidosis and Systemic Involvement
Sarcoidosis is a multiorgan, systemic, granulomatous disease that most commonly affects the cutaneous, pulmonary, ocular, and cardiac organ systems. Cutaneous involvement occurs in approximately 20% to 35% of patients, with approximately 25% of patients demonstrating only dermatologic findings.1 Cutaneous sarcoidosis can have a highly variable presentation. Ichthyosiform sarcoidosis (IS) is a rare form of this disease that has been described as presenting as polygonal adherent scales.2 It often is associated with internal organ involvement. We present a case of IS without any organ system involvement at the time of diagnosis. A review of the English-language literature was performed to ascertain the internal organ associations most commonly reported with IS.
Case Report
A 66-year-old black woman presented to dermatology with dark scaly patches noted by her primary care physician to be present on both of the lower extremities. The patient believed they were present for at least 4 years. She described dark spots confined to the lower legs that had gradually increased in size. Review of systems was negative for fever, chills, night sweats, weight loss, vision changes, cough, dyspnea, and joint pains, and there was no history of either personal or familial cutaneous diseases.
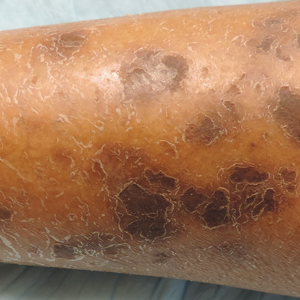
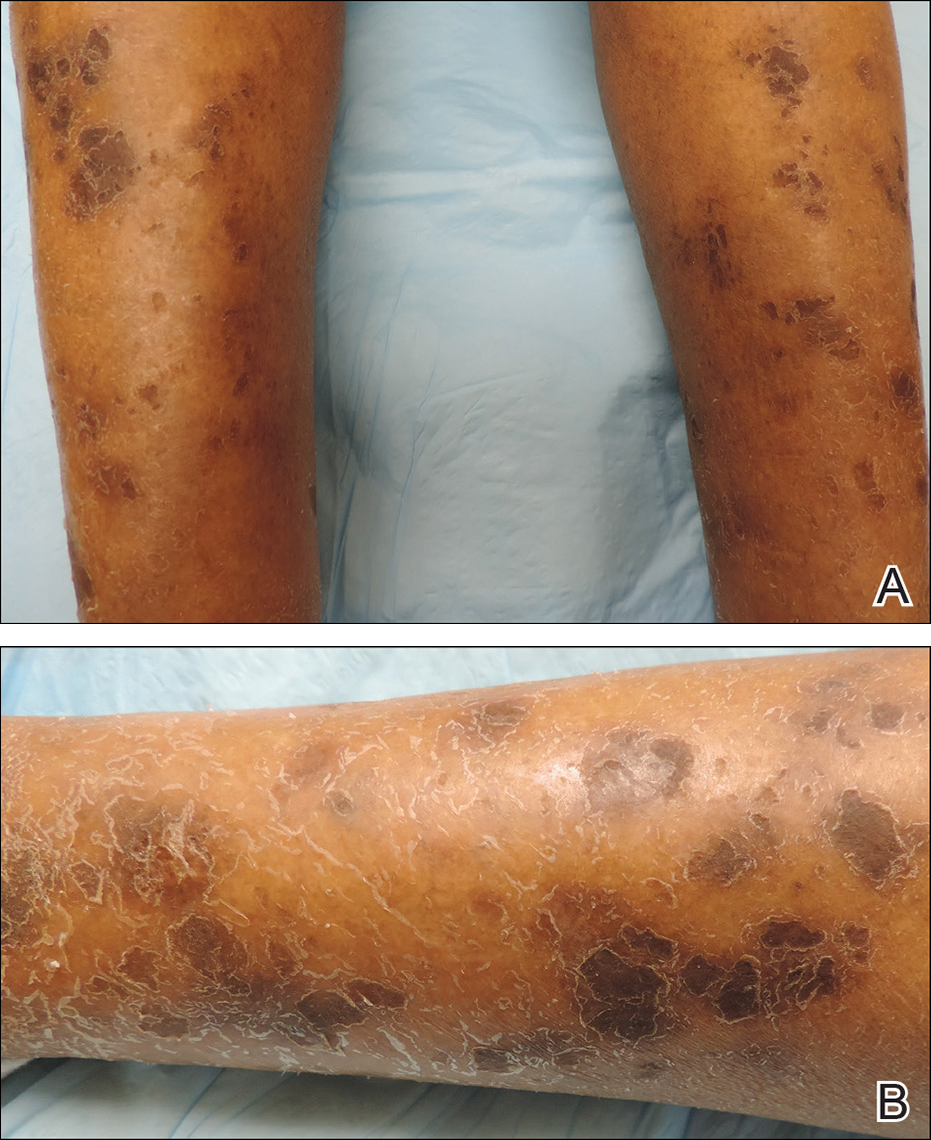
Physical examination revealed cutaneous patches of thin white scale with a sharp edge in arciform patterns on the lower extremities. Several of these patches were hyperpigmented and xerotic in appearance (Figure 1). The patches were limited to the lower legs, with no other lesions noted.
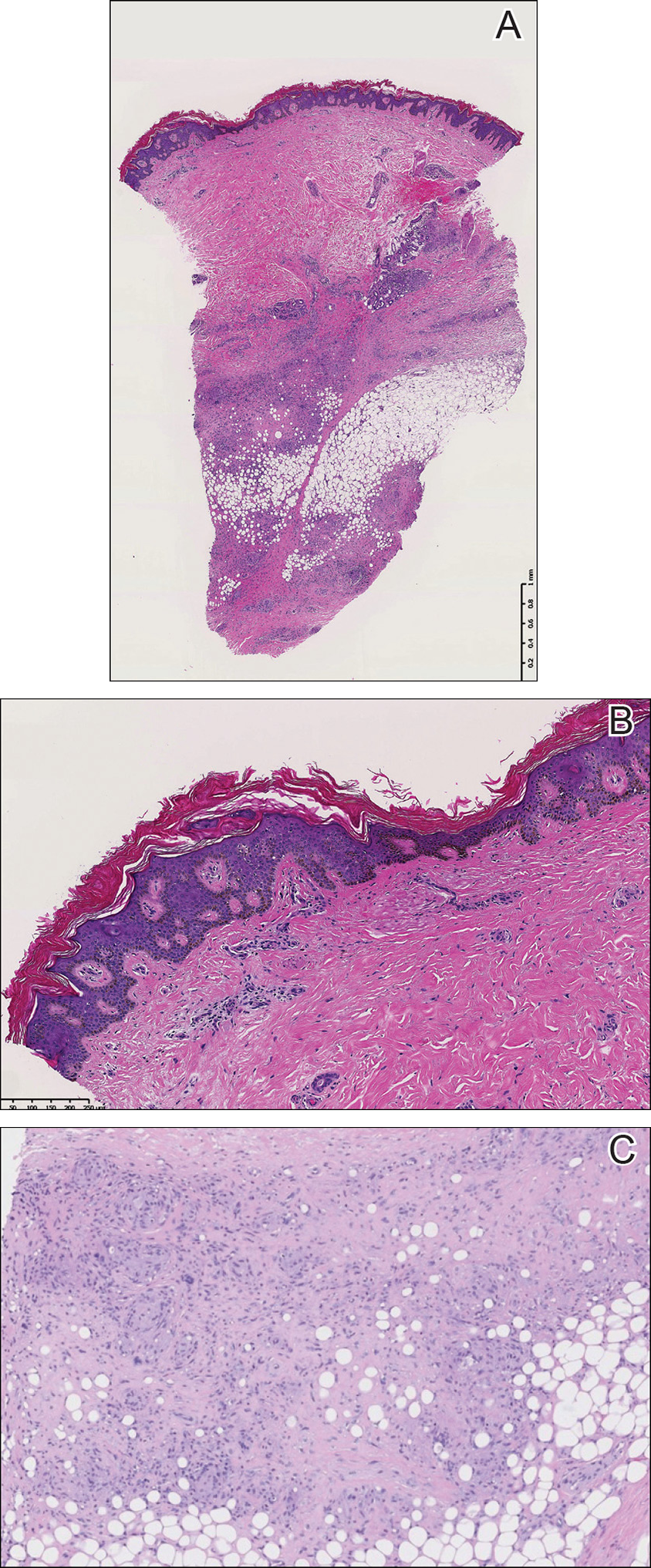
A punch biopsy of the skin on the right lower leg was performed. Histopathologic analysis showed epidermal compact hyperkeratosis with deep granulomatous infiltration into the subcutaneous tissue (Figures 2A and 2B). At high power, these granulomas were noted to be noncaseating naked granulomas composed of epithelioid histiocytes surrounded by sparse lymphocytic inflammation (Figure 2C). Special stains including acid-fast bacilli, Fite, and periodic acid–Schiff were negative. The diagnosis of IS was made based on clinical presentation and primarily by histopathologic analysis.
The patient’s cutaneous lesions were treated with fluocinonide ointment 0.05% twice daily. Although she did not notice a dramatic improvement in the plaques, they stabilized in size. Her primary care physician was notified and advised to begin a workup for involvement of other organ systems by sarcoidosis. Her initial evaluation, which included a chest radiograph and electrocardiogram, were unremarkable. Despite multiple attempts to persuade the patient to return for further follow-up, neither dermatology nor her primary care physician were able to complete a full workup.
Comment
Etiology
Although there are several theories regarding the etiology of sarcoidosis, the exact cause remains unknown. The body’s immune response, infectious agents, genetics, and the environment have all been thought to play a role. It has been well established that helper T cell (TH1) production of interferon and increased levels of tumor necrosis factor propagate the inflammatory response seen in sarcoidosis.3 More recently, TH17 cells have been found in cutaneous lesions, bronchoalveolar lavage samples, and the blood of patients with sarcoidosis, especially in those with active disease progression.3 Infectious agents such as mycobacteria and propionibacteria DNA or RNA also have been found in sarcoid samples.4 Several HLA-DRB1 variants have been associated with an increased incidence of sarcoidosis.5
Presentation
Characteristic dermatologic findings of sarcoidosis include macules, papules, nodules, and plaques located on the face, especially the nose, cheeks, and ears, and on the shins or ankles, as well as similar lesions around tattoos or scars. Sarcoid lesions also have been described as angiolupoid, lichenoid, annular, verrucous, ulcerative, and psoriasiform. Here we present an example of the uncommon type, ichthyosiform. Ichthyosiform sarcoidosis is a rare variant described primarily in dark-skinned individuals, a finding supported by both our case and prior reports. Most reported cases have described IS lesions as having a pasted-on appearance, with adherent centers on the extensor surfaces of the lower extremities, head, and/or neck.6 Our case follows this descriptive pattern previously reported with adherent patches limited to the lower extremities.
Histopathology
The key histopathologic finding is the presence of noncaseating granulomas on biopsy. Sarcoid “specific” lesions rest on the identification of the noncaseating granulomas, while “nonspecific” lesions such as erythema nodosum fail to demonstrate this finding.1
Systemic Involvement
The IS type is believed to be an excellent marker for systemic disease, with approximately 95% of reported cases having some form of systemic illness.6 Acquired ichthyosis should warrant further investigation for systemic disease. Early recognition could be beneficial for the patient because the ichthyosiform type is believed to precede the diagnosis of systemic disease in most cases by a median of 3 months.6
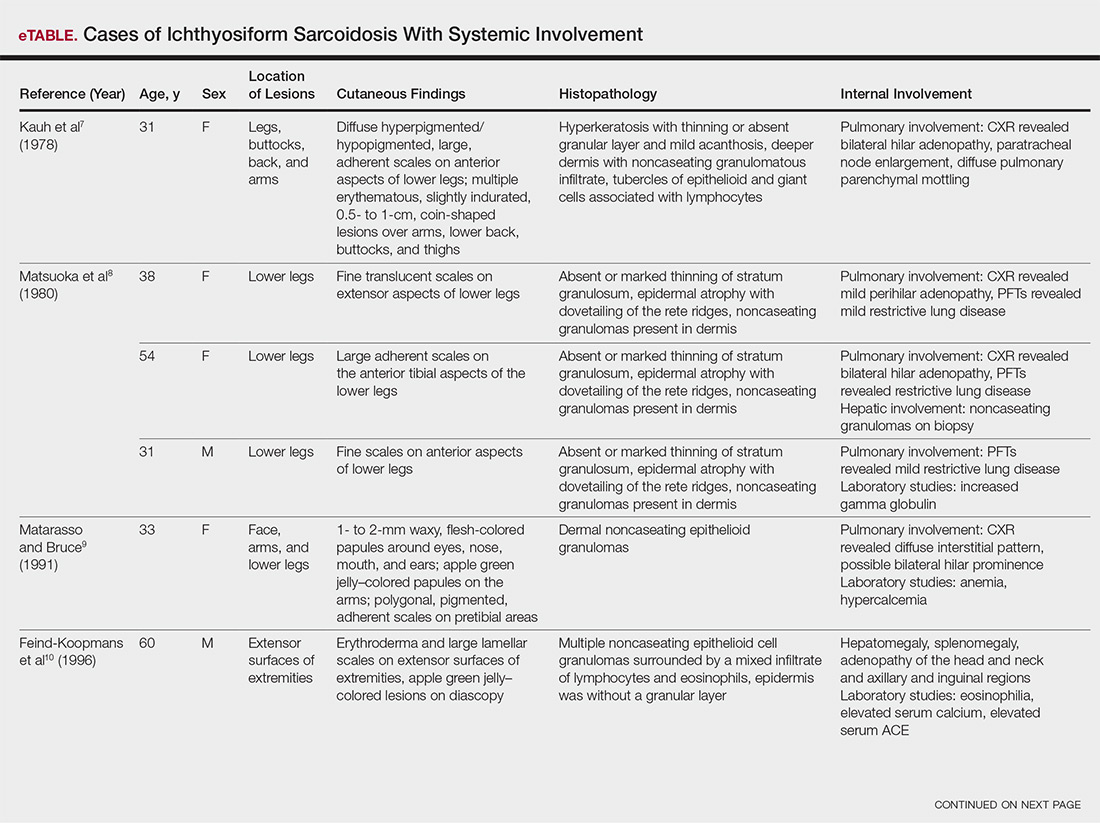
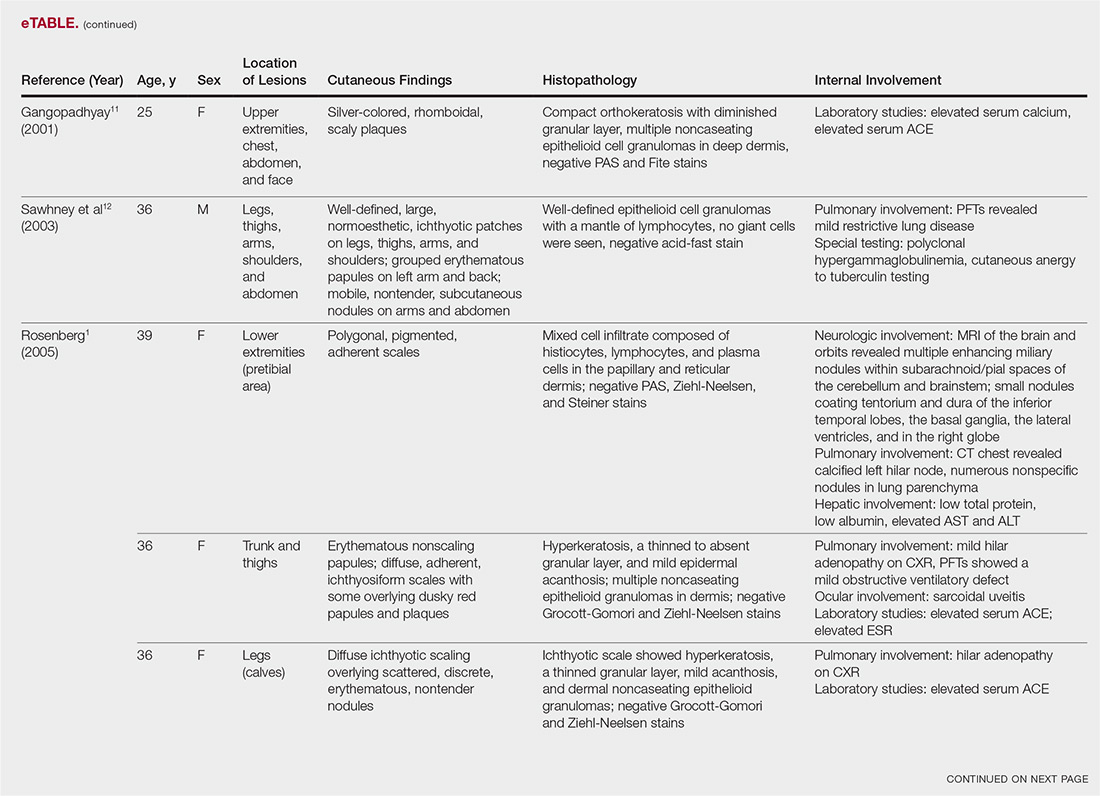
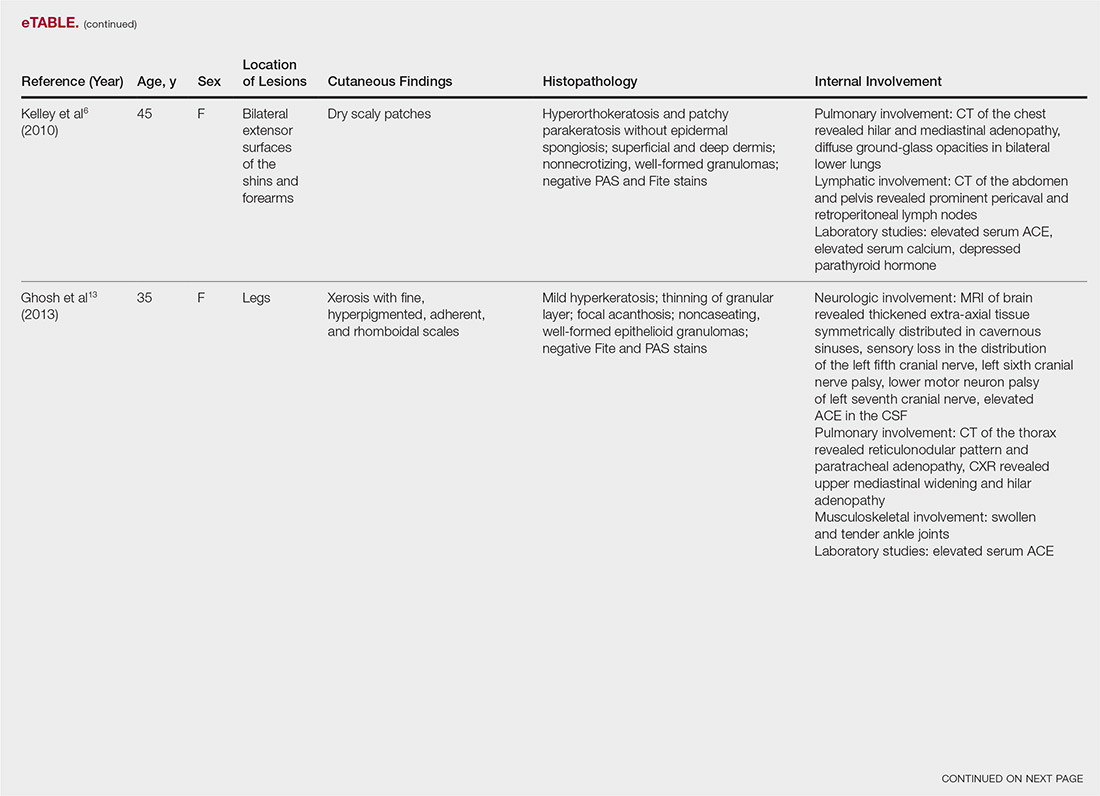
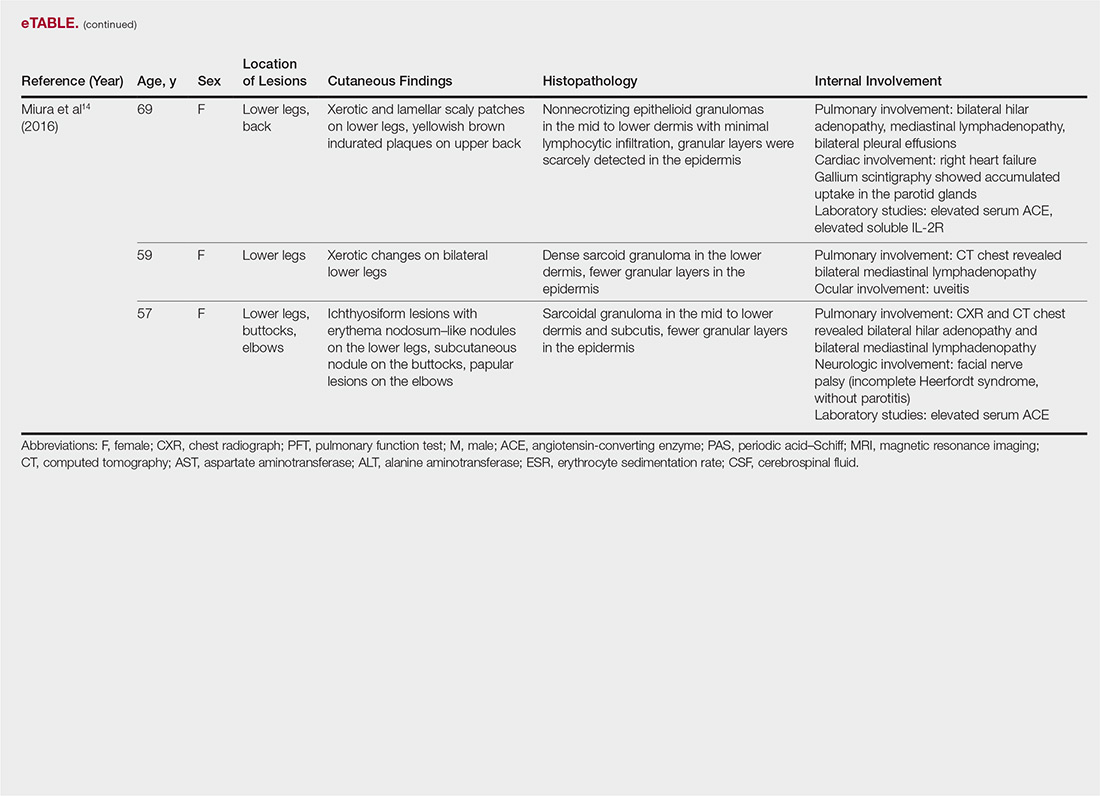
The most common site of internal sarcoid involvement is the lungs, but the lymph nodes, eyes, liver, spleen, heart, and central nervous system also can be involved. Patients can present with nonspecific symptoms such as erythema nodosum in the skin, dyspnea, cough, chest pain, vision changes, enlarged lymph nodes, headaches, joint pain, fever, fatigue, weight loss, and malaise. According to a PubMed search of articles indexed for MEDLINE using the term ichthyosiform sarcoidosis, 16 cases have been reported in the English-language literature (eTable).1,6-14 Of these 16 cases, 3 involved men and 13 involved women. The median age of a patient diagnosed with IS was 37 years. The respiratory system was found to be the most common organ system involved (14 of 16 patients), with hilar adenopathy and restrictive lung disease being the most common findings. Neurologic findings and hepatic involvement also were seen in 3 and 3 patients, respectively. Eight of 16 cases had an elevated serum angiotensin-converting enzyme level. Details of systemic involvement in other cases of IS are listed in the eTable.
Management
Most patients are given topical corticosteroids for their cutaneous lesions, but patients with systemic involvement will likely need some type of systemic immunosuppressive therapy to control their disease. Systemic therapy often is warranted in IS because of reports of rapid progression. Our case differs from these prior reports in the relative stability of the disease at the last patient encounter. Systemic treatment commonly includes oral corticosteroids such as prednisone. Other options, such as hydroxychloroquine, methotrexate, azathioprine, pentoxifylline, thalidomide, cyclophosphamide, cyclosporine, and infliximab, can be considered if other treatments fail.13 Ichthyosiform sarcoidosis patients should continue to have regular follow-up to monitor for disease progression.
Differential
When evaluating an acquired ichthyosis, dermatologists can consider other associations such as Hodgkin disease, hypothyroidism, multiple myeloma, carcinomatosis, and chronic malnutrition.1 Skin biopsy demonstrating granuloma formation also is not specific for sarcoidosis. Other etiologies, such as autoimmune diseases, immunodeficiency disorders, infections, foreign body granulomas, neoplasms, and drug reactions, should be considered.15 All patients with acquired ichthyosis should undergo a thorough evaluation for internal involvement.
Conclusion
We presented a case of IS, a rare type of sarcoidosis commonly associated with further internal involvement of the respiratory, nervous, or hepatic organ systems. Recognition of an acquired form of ichthyosis and its potential disease associations, including sarcoidosis, is important to improve early detection of any internal disease, allowing prompt initiation of treatment.
- Rosenberg B. Ichthyosiform sarcoidosis. Dermatol Online J. 2005;11:15.
- Banse-Kupin L, Pelachyk JM. Ichthyosiform sarcoidosis: report of two cases and review of the literature. J Am Acad Dermatol. 1987;17:616-620.
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416.
- Celada LJ, Hawkins C, Drake WP. The etiologic role of infectious antigens in sarcoidosis pathogenesis. Clin Chest Med. 2015;36:561-568.
- Fingerlin TE, Hamzeh N, Maier LA. Genetics of sarcoidosis. Clin Chest Med. 2015;36:569-584.
- Kelley BP, George DE, LeLeux TM, et al. Ichthyosiform sarcoidosis: a case report and review of the literature. Dermatol Online J. 2010;16:5.
- Kauh YC, Goody HE, Luscombe HA. Ichthyosiform sarcoidosis. Arch Dermatol. 1978;114:100-101.
- Matsuoka LY, LeVine M, Glasser S, et al. Ichthyosiform sarcoid. Cutis. 1980;25:188-189.
- Matarasso SL, Bruce S. Ichthyosiform sarcoidosis: report of a case. Cutis. 1991;47:405-408.
- Feind-Koopmans AG, Lucker GP, van de Kerkhof PC. Acquired ichthyosiform erythroderma and sarcoidosis. J Am Acad Dermatol. 1996;35:826-828.
- Gangopadhyay AK. Ichthyosiform sarcoidosis. Indian J Dermatol Venereol Leprol. 2001;67:91-92.
- Sawhney M, Sharma YK, Gera V, et al. Ichthyosiform sarcoidosis following chemotherapy of Hodgkin’s disease. Indian J Dermatol Venereol Leprol. 2003;69:220-222.
- Ghosh UC, Ghosh SK, Hazra K, et al. Ichthyosiform sarcoidosis revisited. Indian J Dermatol Venereol Leprol. 2013;79:795-798.
- Miura T, Kato Y, Yamamoto T. Ichthyosiform sarcoidosis: report of three cases from Japan and literature review. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:392-397.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
Sarcoidosis is a multiorgan, systemic, granulomatous disease that most commonly affects the cutaneous, pulmonary, ocular, and cardiac organ systems. Cutaneous involvement occurs in approximately 20% to 35% of patients, with approximately 25% of patients demonstrating only dermatologic findings.1 Cutaneous sarcoidosis can have a highly variable presentation. Ichthyosiform sarcoidosis (IS) is a rare form of this disease that has been described as presenting as polygonal adherent scales.2 It often is associated with internal organ involvement. We present a case of IS without any organ system involvement at the time of diagnosis. A review of the English-language literature was performed to ascertain the internal organ associations most commonly reported with IS.
Case Report
A 66-year-old black woman presented to dermatology with dark scaly patches noted by her primary care physician to be present on both of the lower extremities. The patient believed they were present for at least 4 years. She described dark spots confined to the lower legs that had gradually increased in size. Review of systems was negative for fever, chills, night sweats, weight loss, vision changes, cough, dyspnea, and joint pains, and there was no history of either personal or familial cutaneous diseases.
Physical examination revealed cutaneous patches of thin white scale with a sharp edge in arciform patterns on the lower extremities. Several of these patches were hyperpigmented and xerotic in appearance (Figure 1). The patches were limited to the lower legs, with no other lesions noted.

A punch biopsy of the skin on the right lower leg was performed. Histopathologic analysis showed epidermal compact hyperkeratosis with deep granulomatous infiltration into the subcutaneous tissue (Figures 2A and 2B). At high power, these granulomas were noted to be noncaseating naked granulomas composed of epithelioid histiocytes surrounded by sparse lymphocytic inflammation (Figure 2C). Special stains including acid-fast bacilli, Fite, and periodic acid–Schiff were negative. The diagnosis of IS was made based on clinical presentation and primarily by histopathologic analysis.

The patient’s cutaneous lesions were treated with fluocinonide ointment 0.05% twice daily. Although she did not notice a dramatic improvement in the plaques, they stabilized in size. Her primary care physician was notified and advised to begin a workup for involvement of other organ systems by sarcoidosis. Her initial evaluation, which included a chest radiograph and electrocardiogram, were unremarkable. Despite multiple attempts to persuade the patient to return for further follow-up, neither dermatology nor her primary care physician were able to complete a full workup.
Comment
Etiology
Although there are several theories regarding the etiology of sarcoidosis, the exact cause remains unknown. The body’s immune response, infectious agents, genetics, and the environment have all been thought to play a role. It has been well established that helper T cell (TH1) production of interferon and increased levels of tumor necrosis factor propagate the inflammatory response seen in sarcoidosis.3 More recently, TH17 cells have been found in cutaneous lesions, bronchoalveolar lavage samples, and the blood of patients with sarcoidosis, especially in those with active disease progression.3 Infectious agents such as mycobacteria and propionibacteria DNA or RNA also have been found in sarcoid samples.4 Several HLA-DRB1 variants have been associated with an increased incidence of sarcoidosis.5
Presentation
Characteristic dermatologic findings of sarcoidosis include macules, papules, nodules, and plaques located on the face, especially the nose, cheeks, and ears, and on the shins or ankles, as well as similar lesions around tattoos or scars. Sarcoid lesions also have been described as angiolupoid, lichenoid, annular, verrucous, ulcerative, and psoriasiform. Here we present an example of the uncommon type, ichthyosiform. Ichthyosiform sarcoidosis is a rare variant described primarily in dark-skinned individuals, a finding supported by both our case and prior reports. Most reported cases have described IS lesions as having a pasted-on appearance, with adherent centers on the extensor surfaces of the lower extremities, head, and/or neck.6 Our case follows this descriptive pattern previously reported with adherent patches limited to the lower extremities.
Histopathology
The key histopathologic finding is the presence of noncaseating granulomas on biopsy. Sarcoid “specific” lesions rest on the identification of the noncaseating granulomas, while “nonspecific” lesions such as erythema nodosum fail to demonstrate this finding.1
Systemic Involvement
The IS type is believed to be an excellent marker for systemic disease, with approximately 95% of reported cases having some form of systemic illness.6 Acquired ichthyosis should warrant further investigation for systemic disease. Early recognition could be beneficial for the patient because the ichthyosiform type is believed to precede the diagnosis of systemic disease in most cases by a median of 3 months.6
The most common site of internal sarcoid involvement is the lungs, but the lymph nodes, eyes, liver, spleen, heart, and central nervous system also can be involved. Patients can present with nonspecific symptoms such as erythema nodosum in the skin, dyspnea, cough, chest pain, vision changes, enlarged lymph nodes, headaches, joint pain, fever, fatigue, weight loss, and malaise. According to a PubMed search of articles indexed for MEDLINE using the term ichthyosiform sarcoidosis, 16 cases have been reported in the English-language literature (eTable).1,6-14 Of these 16 cases, 3 involved men and 13 involved women. The median age of a patient diagnosed with IS was 37 years. The respiratory system was found to be the most common organ system involved (14 of 16 patients), with hilar adenopathy and restrictive lung disease being the most common findings. Neurologic findings and hepatic involvement also were seen in 3 and 3 patients, respectively. Eight of 16 cases had an elevated serum angiotensin-converting enzyme level. Details of systemic involvement in other cases of IS are listed in the eTable.
Management
Most patients are given topical corticosteroids for their cutaneous lesions, but patients with systemic involvement will likely need some type of systemic immunosuppressive therapy to control their disease. Systemic therapy often is warranted in IS because of reports of rapid progression. Our case differs from these prior reports in the relative stability of the disease at the last patient encounter. Systemic treatment commonly includes oral corticosteroids such as prednisone. Other options, such as hydroxychloroquine, methotrexate, azathioprine, pentoxifylline, thalidomide, cyclophosphamide, cyclosporine, and infliximab, can be considered if other treatments fail.13 Ichthyosiform sarcoidosis patients should continue to have regular follow-up to monitor for disease progression.
Differential
When evaluating an acquired ichthyosis, dermatologists can consider other associations such as Hodgkin disease, hypothyroidism, multiple myeloma, carcinomatosis, and chronic malnutrition.1 Skin biopsy demonstrating granuloma formation also is not specific for sarcoidosis. Other etiologies, such as autoimmune diseases, immunodeficiency disorders, infections, foreign body granulomas, neoplasms, and drug reactions, should be considered.15 All patients with acquired ichthyosis should undergo a thorough evaluation for internal involvement.
Conclusion
We presented a case of IS, a rare type of sarcoidosis commonly associated with further internal involvement of the respiratory, nervous, or hepatic organ systems. Recognition of an acquired form of ichthyosis and its potential disease associations, including sarcoidosis, is important to improve early detection of any internal disease, allowing prompt initiation of treatment.
Sarcoidosis is a multiorgan, systemic, granulomatous disease that most commonly affects the cutaneous, pulmonary, ocular, and cardiac organ systems. Cutaneous involvement occurs in approximately 20% to 35% of patients, with approximately 25% of patients demonstrating only dermatologic findings.1 Cutaneous sarcoidosis can have a highly variable presentation. Ichthyosiform sarcoidosis (IS) is a rare form of this disease that has been described as presenting as polygonal adherent scales.2 It often is associated with internal organ involvement. We present a case of IS without any organ system involvement at the time of diagnosis. A review of the English-language literature was performed to ascertain the internal organ associations most commonly reported with IS.
Case Report
A 66-year-old black woman presented to dermatology with dark scaly patches noted by her primary care physician to be present on both of the lower extremities. The patient believed they were present for at least 4 years. She described dark spots confined to the lower legs that had gradually increased in size. Review of systems was negative for fever, chills, night sweats, weight loss, vision changes, cough, dyspnea, and joint pains, and there was no history of either personal or familial cutaneous diseases.
Physical examination revealed cutaneous patches of thin white scale with a sharp edge in arciform patterns on the lower extremities. Several of these patches were hyperpigmented and xerotic in appearance (Figure 1). The patches were limited to the lower legs, with no other lesions noted.

A punch biopsy of the skin on the right lower leg was performed. Histopathologic analysis showed epidermal compact hyperkeratosis with deep granulomatous infiltration into the subcutaneous tissue (Figures 2A and 2B). At high power, these granulomas were noted to be noncaseating naked granulomas composed of epithelioid histiocytes surrounded by sparse lymphocytic inflammation (Figure 2C). Special stains including acid-fast bacilli, Fite, and periodic acid–Schiff were negative. The diagnosis of IS was made based on clinical presentation and primarily by histopathologic analysis.

The patient’s cutaneous lesions were treated with fluocinonide ointment 0.05% twice daily. Although she did not notice a dramatic improvement in the plaques, they stabilized in size. Her primary care physician was notified and advised to begin a workup for involvement of other organ systems by sarcoidosis. Her initial evaluation, which included a chest radiograph and electrocardiogram, were unremarkable. Despite multiple attempts to persuade the patient to return for further follow-up, neither dermatology nor her primary care physician were able to complete a full workup.
Comment
Etiology
Although there are several theories regarding the etiology of sarcoidosis, the exact cause remains unknown. The body’s immune response, infectious agents, genetics, and the environment have all been thought to play a role. It has been well established that helper T cell (TH1) production of interferon and increased levels of tumor necrosis factor propagate the inflammatory response seen in sarcoidosis.3 More recently, TH17 cells have been found in cutaneous lesions, bronchoalveolar lavage samples, and the blood of patients with sarcoidosis, especially in those with active disease progression.3 Infectious agents such as mycobacteria and propionibacteria DNA or RNA also have been found in sarcoid samples.4 Several HLA-DRB1 variants have been associated with an increased incidence of sarcoidosis.5
Presentation
Characteristic dermatologic findings of sarcoidosis include macules, papules, nodules, and plaques located on the face, especially the nose, cheeks, and ears, and on the shins or ankles, as well as similar lesions around tattoos or scars. Sarcoid lesions also have been described as angiolupoid, lichenoid, annular, verrucous, ulcerative, and psoriasiform. Here we present an example of the uncommon type, ichthyosiform. Ichthyosiform sarcoidosis is a rare variant described primarily in dark-skinned individuals, a finding supported by both our case and prior reports. Most reported cases have described IS lesions as having a pasted-on appearance, with adherent centers on the extensor surfaces of the lower extremities, head, and/or neck.6 Our case follows this descriptive pattern previously reported with adherent patches limited to the lower extremities.
Histopathology
The key histopathologic finding is the presence of noncaseating granulomas on biopsy. Sarcoid “specific” lesions rest on the identification of the noncaseating granulomas, while “nonspecific” lesions such as erythema nodosum fail to demonstrate this finding.1
Systemic Involvement
The IS type is believed to be an excellent marker for systemic disease, with approximately 95% of reported cases having some form of systemic illness.6 Acquired ichthyosis should warrant further investigation for systemic disease. Early recognition could be beneficial for the patient because the ichthyosiform type is believed to precede the diagnosis of systemic disease in most cases by a median of 3 months.6
The most common site of internal sarcoid involvement is the lungs, but the lymph nodes, eyes, liver, spleen, heart, and central nervous system also can be involved. Patients can present with nonspecific symptoms such as erythema nodosum in the skin, dyspnea, cough, chest pain, vision changes, enlarged lymph nodes, headaches, joint pain, fever, fatigue, weight loss, and malaise. According to a PubMed search of articles indexed for MEDLINE using the term ichthyosiform sarcoidosis, 16 cases have been reported in the English-language literature (eTable).1,6-14 Of these 16 cases, 3 involved men and 13 involved women. The median age of a patient diagnosed with IS was 37 years. The respiratory system was found to be the most common organ system involved (14 of 16 patients), with hilar adenopathy and restrictive lung disease being the most common findings. Neurologic findings and hepatic involvement also were seen in 3 and 3 patients, respectively. Eight of 16 cases had an elevated serum angiotensin-converting enzyme level. Details of systemic involvement in other cases of IS are listed in the eTable.
Management
Most patients are given topical corticosteroids for their cutaneous lesions, but patients with systemic involvement will likely need some type of systemic immunosuppressive therapy to control their disease. Systemic therapy often is warranted in IS because of reports of rapid progression. Our case differs from these prior reports in the relative stability of the disease at the last patient encounter. Systemic treatment commonly includes oral corticosteroids such as prednisone. Other options, such as hydroxychloroquine, methotrexate, azathioprine, pentoxifylline, thalidomide, cyclophosphamide, cyclosporine, and infliximab, can be considered if other treatments fail.13 Ichthyosiform sarcoidosis patients should continue to have regular follow-up to monitor for disease progression.
Differential
When evaluating an acquired ichthyosis, dermatologists can consider other associations such as Hodgkin disease, hypothyroidism, multiple myeloma, carcinomatosis, and chronic malnutrition.1 Skin biopsy demonstrating granuloma formation also is not specific for sarcoidosis. Other etiologies, such as autoimmune diseases, immunodeficiency disorders, infections, foreign body granulomas, neoplasms, and drug reactions, should be considered.15 All patients with acquired ichthyosis should undergo a thorough evaluation for internal involvement.
Conclusion
We presented a case of IS, a rare type of sarcoidosis commonly associated with further internal involvement of the respiratory, nervous, or hepatic organ systems. Recognition of an acquired form of ichthyosis and its potential disease associations, including sarcoidosis, is important to improve early detection of any internal disease, allowing prompt initiation of treatment.
- Rosenberg B. Ichthyosiform sarcoidosis. Dermatol Online J. 2005;11:15.
- Banse-Kupin L, Pelachyk JM. Ichthyosiform sarcoidosis: report of two cases and review of the literature. J Am Acad Dermatol. 1987;17:616-620.
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416.
- Celada LJ, Hawkins C, Drake WP. The etiologic role of infectious antigens in sarcoidosis pathogenesis. Clin Chest Med. 2015;36:561-568.
- Fingerlin TE, Hamzeh N, Maier LA. Genetics of sarcoidosis. Clin Chest Med. 2015;36:569-584.
- Kelley BP, George DE, LeLeux TM, et al. Ichthyosiform sarcoidosis: a case report and review of the literature. Dermatol Online J. 2010;16:5.
- Kauh YC, Goody HE, Luscombe HA. Ichthyosiform sarcoidosis. Arch Dermatol. 1978;114:100-101.
- Matsuoka LY, LeVine M, Glasser S, et al. Ichthyosiform sarcoid. Cutis. 1980;25:188-189.
- Matarasso SL, Bruce S. Ichthyosiform sarcoidosis: report of a case. Cutis. 1991;47:405-408.
- Feind-Koopmans AG, Lucker GP, van de Kerkhof PC. Acquired ichthyosiform erythroderma and sarcoidosis. J Am Acad Dermatol. 1996;35:826-828.
- Gangopadhyay AK. Ichthyosiform sarcoidosis. Indian J Dermatol Venereol Leprol. 2001;67:91-92.
- Sawhney M, Sharma YK, Gera V, et al. Ichthyosiform sarcoidosis following chemotherapy of Hodgkin’s disease. Indian J Dermatol Venereol Leprol. 2003;69:220-222.
- Ghosh UC, Ghosh SK, Hazra K, et al. Ichthyosiform sarcoidosis revisited. Indian J Dermatol Venereol Leprol. 2013;79:795-798.
- Miura T, Kato Y, Yamamoto T. Ichthyosiform sarcoidosis: report of three cases from Japan and literature review. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:392-397.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
- Rosenberg B. Ichthyosiform sarcoidosis. Dermatol Online J. 2005;11:15.
- Banse-Kupin L, Pelachyk JM. Ichthyosiform sarcoidosis: report of two cases and review of the literature. J Am Acad Dermatol. 1987;17:616-620.
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416.
- Celada LJ, Hawkins C, Drake WP. The etiologic role of infectious antigens in sarcoidosis pathogenesis. Clin Chest Med. 2015;36:561-568.
- Fingerlin TE, Hamzeh N, Maier LA. Genetics of sarcoidosis. Clin Chest Med. 2015;36:569-584.
- Kelley BP, George DE, LeLeux TM, et al. Ichthyosiform sarcoidosis: a case report and review of the literature. Dermatol Online J. 2010;16:5.
- Kauh YC, Goody HE, Luscombe HA. Ichthyosiform sarcoidosis. Arch Dermatol. 1978;114:100-101.
- Matsuoka LY, LeVine M, Glasser S, et al. Ichthyosiform sarcoid. Cutis. 1980;25:188-189.
- Matarasso SL, Bruce S. Ichthyosiform sarcoidosis: report of a case. Cutis. 1991;47:405-408.
- Feind-Koopmans AG, Lucker GP, van de Kerkhof PC. Acquired ichthyosiform erythroderma and sarcoidosis. J Am Acad Dermatol. 1996;35:826-828.
- Gangopadhyay AK. Ichthyosiform sarcoidosis. Indian J Dermatol Venereol Leprol. 2001;67:91-92.
- Sawhney M, Sharma YK, Gera V, et al. Ichthyosiform sarcoidosis following chemotherapy of Hodgkin’s disease. Indian J Dermatol Venereol Leprol. 2003;69:220-222.
- Ghosh UC, Ghosh SK, Hazra K, et al. Ichthyosiform sarcoidosis revisited. Indian J Dermatol Venereol Leprol. 2013;79:795-798.
- Miura T, Kato Y, Yamamoto T. Ichthyosiform sarcoidosis: report of three cases from Japan and literature review. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:392-397.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
Practice Points
- Ichthyosiform sarcoidosis is a rare form of sarcoidosis that presents as polygonal adherent scales.
- Ichthyosiform sarcoidosis is commonly associated with pulmonary, neurologic, and hepatic involvement.
- Acquired ichthyosis should warrant further investigation for systemic disease.