User login
Psoriasis is a common immune-mediated papulosquamous skin disease with a generally chronic course. Impairments in quality of life (QOL) and psychological morbidity in the form of anxiety and depression have been reported.1 Because psoriasis is not known to directly affect the central nervous system, the associated psychiatric morbidity is likely caused by the complex interplay of the stress, physical discomfort, and possible disfigurement inherent to psoriasis, as well as the emotional response to the condition mediated by the patient’s personality, emotional and cognitive state, and other social factors (eg, self-stigma and perceived stigma, lack of knowledge about the illness in the patient and in the community and family, lack of resources and support).2 Because a variety of methodologies have been used in research on the association of psoriasis with psychiatric morbidity, it is not easy to compare findings. Most studies have assessed psychiatric symptoms rather than findings from psychiatric diagnostic instruments.3 The diagnosis of psychiatric disorders in patients with psoriasis rather than focusing on symptoms alone is likely to be more useful in generating scientific epidemiologic data and also would serve as a guide in making treatment and policy decisions. Validated clinician-rated instruments are useful in generating these data. However, psychiatric diagnoses are often missed by dermatologists, which may have an adverse impact on eventual outcomes in psoriasis patients.4,5 Patient-assessed diagnostic instruments may help dermatologists overcome this problem.
This study investigated the prevalence and determinants of psychiatric disorders in a cohort of psoriasis patients in North India using both patient self-assessment and clinician-administered instruments.
Methods
Study Participants
The study was conducted from January 2013 to November 2013 at the Postgraduate Institute of Medical Education and Research, a tertiary-level teaching hospital in Chandigarh, India, which serves the population of a large geographic area in North India. Clearance for this study was obtained from the institute ethics committee.
Patients with chronic plaque psoriasis who presented consecutively to the outpatient clinic of the Departments of Dermatology, Venereology, and Leprology during the study period were approached for participation. Written informed consent was obtained from all participants. Inclusion criteria were the ability to read the self-assessment questionnaires, and no financial compensation was offered for inclusion in the study. Patients with psoriatic arthritis as well as erythrodermic and pustular variants of psoriasis were excluded. Exclusion criteria also included patients with known diabetes mellitus, cardiovascular disease, chronic respiratory ailments, or other notable systemic comorbidities; however, patients did not undergo biochemical testing.
Assessments
A 2-stage methodology was employed. In the first stage of the assessment, sociodemographic and clinical data were recorded. Thereafter, psychiatric symptoms and morbidity were assessed using the patient health questionnaire (PHQ).6 Quality of life was assessed using the dermatology life quality index (DLQI).7 Both tools were based on patient self-assessment. Study participants could seek assistance from the clinician in completing the questionnaires, if needed. Psoriasis severity was evaluated by the clinician using the psoriasis area severity index (PASI) score.8
In the second stage of the assessment, participants underwent subsequent evaluation by a psychiatrist who was blinded to the results of the first assessments. All participants were screened using the Mini international neuropsychiatric interview (MINI)9 and a formal psychiatric diagnosis was made. In subsequent analyses, we considered psychiatric diagnoses as generated with MINI as the gold standard against which other results were compared.
A participant was considered positive for psychiatric morbidity if he/she was positive for at least 1 PHQ or MINI diagnosis.
To assess for concordance between the 2 diagnostic instruments, the following diagnostic groups were compared against each other: (1) MINI depressive disorders (DDs)(ie, major depressive episode, current and recurrent; dysthymia) versus PHQ depressive disorders (ie, major DDs and other DDs); (2) MINI anxiety disorders (ie, panic disorder and generalized anxiety disorder) versus PHQ anxiety disorders (ie, panic syndrome and other anxiety syndromes); (3) MINI alcohol abuse (ie, alcohol dependence and abuse) versus PHQ alcohol abuse; (4) comorbid disorders if more than 1 diagnosis was made; and (5) any positive score on the MINI suicide module with a response other than not at all on PHQ depression module item 2(i), which deals with thoughts of self-harm and wishing that one was dead. The MINI depressive disorders and PHQ depressive disorders indicate the presence of a clinically significant depressive state and a need for assessment and treatment.
The PHQ can be used to diagnose somatoform disorders, while the MINI cannot be used. Because the somatoform disorders diagnosed were few in number and comorbid with DDs (n=3) and anxiety disorders (n=1), we included these cases with DDs and anxiety disorders, respectively, for purposes of statistical analysis. All data were analyzed using SPSS software.
Results
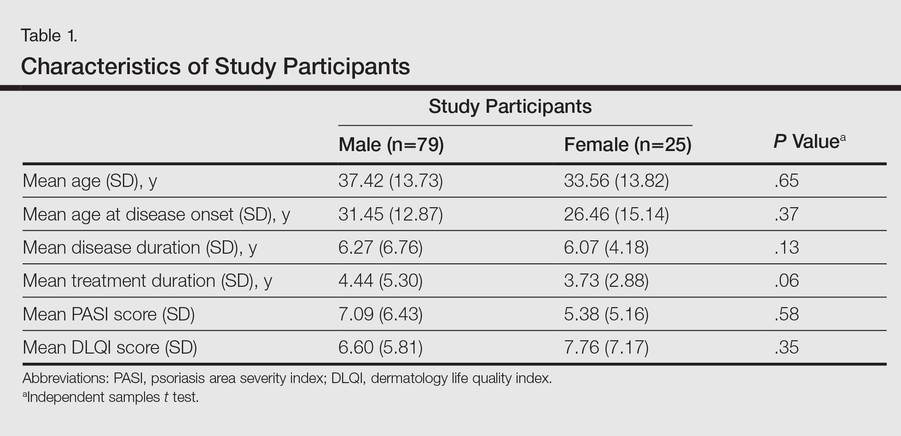
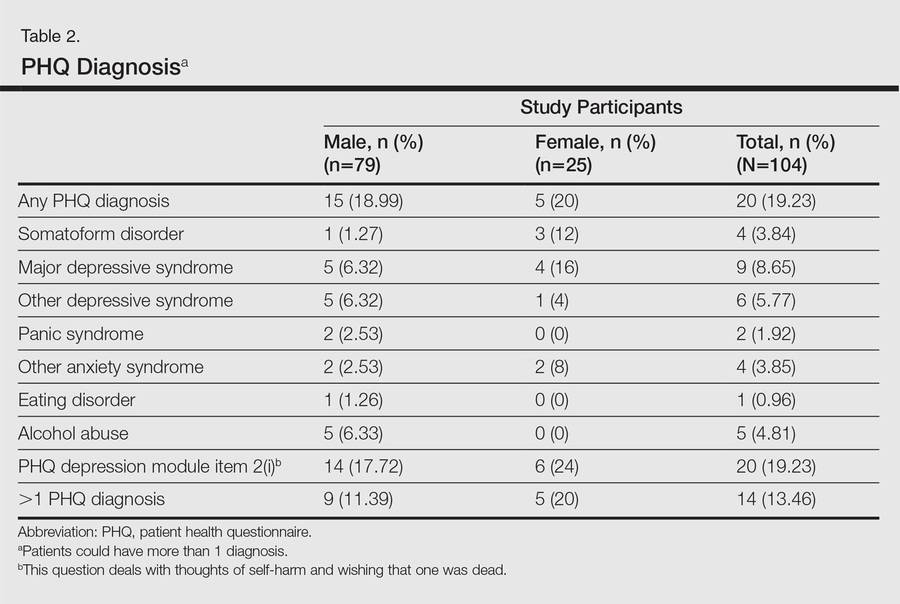
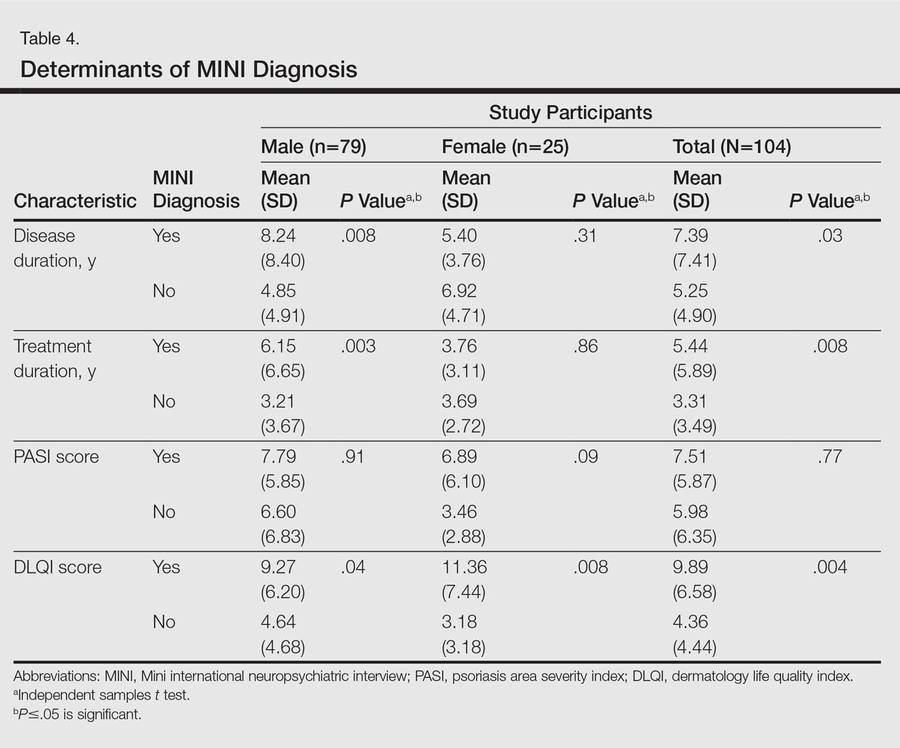
One hundred four participants were included in this study. The sociodemographic, clinical, and diagnostic profiles, as well as the determinants of MINI diagnosis, are provided in Tables 1 through 4. The PASI and DLQI scores indicated that most participants had mild to moderate psoriasis severity.10 The prevalence of alcohol-related disorders was only found in the male subpopulation, which is consistent with the sociocultural context of North India. Psoriasis severity (ie, PASI score) was not found to be a determinant of psychiatric diagnoses in the study population. There was no statistical difference in measures of current clinical status and treatment modality when those with or without any psychiatric diagnoses were compared. When the variables of disease duration, treatment duration, and DLQI were entered into a binary logistic regression with positive status for a MINI diagnosis as a dependent variable indicating the presence of a psychiatric disorder, it was found that the DLQI score was a significant predictor (b=0.19; SE=0.47; χ2=17.92; P<.05). This finding was the same for regression analyses for males and females separately and also for DD as a dependent variable.
Mean DLQI and PASI scores were positively correlated with each other (Pearson r=0.23; P=.01). This relationship was maintained in males (Pearson r=0.24; P=.03) but not in females (Pearson r=0.14; P=.30). The correlations between DLQI and PASI scores and both disease duration and treatment duration were not significant. The Cohen κ values for the interrater reliability analyses done to assess the concordance of the PHQ and MINI diagnostic groups were modest (0.31-0.42), which was true even when MINI depressive disorders without dysthymia and PHQ depressive disorders were compared.
Comment
Studies investigating psychiatric morbidity in psoriasis have varying methodologies, mostly assessing psychiatric symptoms rather than screening for psychiatric disorders.3 In chronic diseases such as psoriasis, there often is an overlap between disease symptoms and common psychiatric disorders (eg, depression).11 Therefore, assessment of symptoms can be misleading. The current study was designed to detect psychiatric disorders in psoriasis patients using both patient self-assessment and clinician-administered instruments. We also investigated the contribution of sociodemographic and clinical variables (eg, psoriasis severity, impairment in QOL) on psychiatric morbidity.
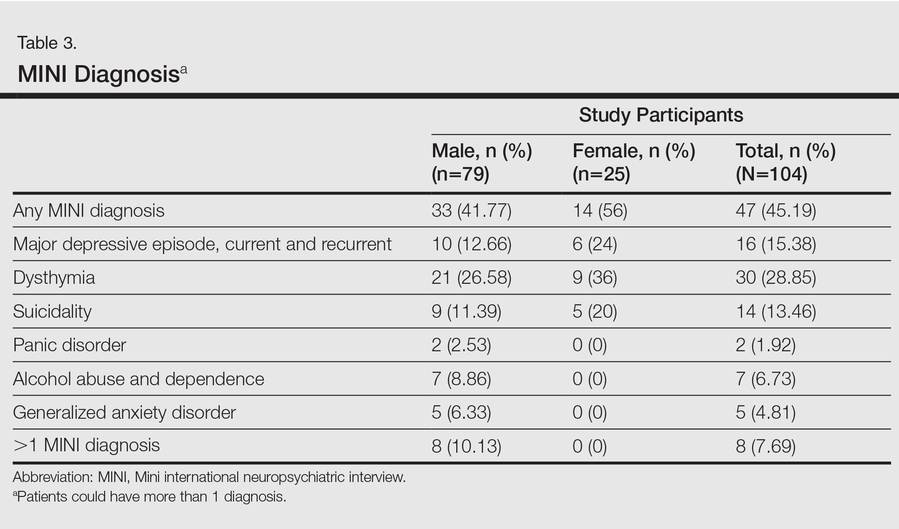
An increased risk for depression, anxiety, and suicidality associated with greater psoriasis severity has been reported.12 The results of the current study indicate that even in a patient population with predominantly mild to moderate psoriasis, psychiatric morbidity, particularly DDs, is common. This finding was seen both on patient self-assessment and clinician-administered evaluations. Earlier studies from this institution and region have reported a lower prevalence of psychiatric disorders in patients with psoriasis (24.7%–36.7%).13-16 However, prior studies were based on assessment of specific symptoms and clinical diagnoses derived from history and mental status examination rather than the administration of more rigorous research diagnostic assessment tools. A systematic review and meta-analysis also revealed a lower prevalence of clinical depression using International Classification of Diseases, Tenth Revision, and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) codes.3 A possible reason for this apparent discrepancy is the fact that psychiatric morbidity in a majority of participants in the current study was constituted by the diagnosis of dysthymia. If the diagnosis of dysthymia is removed from the current analysis, the prevalence of clinical major depressive syndromes is similar to other data. We found that chronic low-grade depression (dysthymia) was the most common diagnosis from the MINI. Lower prevalence was noted in a prior study, but methodology using clinical interviewing may have resulted in an underestimated prevalence.13 It also is possible that chronic low-grade depression in psoriasis patients may be missed or underestimated in comparison to more readily diagnosed and severe depressive syndromes in other studies. However, there is enough evidence to suggest that dysthymia is clinically relevant in the causation of morbidity and disability (eg, physical, psychological, or cognitive impairment) in patients with chronic physical disorders.17 This distinction between clinical major depressive syndrome and dysthymia is important because different treatment methods may be required; the former may warrant treatment with antidepressants in addition to psychosocial treatment modalities (eg, learning to cope with stress, problem-solving techniques), while the latter may benefit predominantly from psychosocial treatment modalities alone.18,19 In the current study, most of the participants who were diagnosed with dysthymia refused treatment with any psychotropic medications but perceived benefit from discussing their problems with a professional. Our results indicated that chronic low-grade depression is more common than more severe major depressive states, and mental health professionals who are well versed in psychosocial treatment modalities should play an integral role in treatment planning for patients with psoriasis.
In the current study, there was only a modest correlation between the results of the patient self-assessment and clinician-administered evaluations, which indicated that psychiatric disorders may not be obvious to clinicians unless specifically investigated, even for some severely depressed and suicidal patients. Given the high prevalence of clinically relevant psychiatric morbidity among psoriasis patients, dermatology professionals should be more sensitive to the possible presence of psychiatric disorders in this patient population and should consider the use of formal screening or other diagnostic tools for detection of depression and anxiety in psoriasis patients.
The main determinant of psychiatric morbidity in our study population was impairment in QOL. Interestingly enough, psoriasis severity was not associated with psychiatric morbidity in our study. Depressive states in patients with chronic physical illnesses are well known and could be due to the chronic stress of illness or impaired QOL, or depression may be a direct effect of the illness and/or treatment on the central nervous system. Psoriasis is not known to have any direct effect on the central nervous system. Our findings suggest that QOL impairment plays an important role in psychiatric morbidity in patients with psoriasis. Even though the DLQI is designed to measure QOL over the preceding week, our findings suggest that impairment in QOL in psoriasis is a manifestation of a more long-term effect of interplay between many factors; the impairment in activities of daily living, disease-related physical discomfort and impaired self-esteem and self-perception, impairments in interpersonal relationships, and the stress of chronicity of illness seem to play an important role. Additionally, variables such as emotional dysfunction, magnitude and site of the area of involvement, nature and magnitude of comorbidities, and complications of illness and coping also may be relevant.20 Although these factors are common in other chronic disorders, psoriasis in particular may predisposepatients to depression due to its unpredictable and relapsing nature, lack of any curative therapy, and the stigmatizing prominent lesions that often are impossible to camouflage. In chronic diseases such as psoriasis, the amelioration of impairment of different aspects of QOL may be more important than mere symptom control.
Our study was limited in that the study population was predominantly male. Fewer females may have consented to participate in the study due to time constraints associated with domestic responsibilities, reluctance to discuss psychological distress, or inability to meet the inclusion criteria (eg, level of education required to read questionnaires). However, there was no significant difference between males and females for sociodemographic variables or diagnoses other than alcohol-related disorders. Our study also had a cross-sectional design and there was no control group, without which it is difficult to assess the true prevalence and determinants of these psychiatric morbidities. Moreover, the sample size was small and did not include enough participants with moderate to severe psoriasis (ie, PASI score ≥10) to be able to detect a correlation between psychiatric morbidities and psoriasis severity. Our findings underline the need for effective screening and integrated management of psychiatric disorders in patients with psoriasis.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Richards HL, Fortune DG, Weidmann A, et al. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol. 2004;151:1227-1233.
- Scharloo M, Kaptein AA, Weinman J, et al. Patients’ illness perceptions and coping as predictors of functional status in psoriasis: a 1-year follow-up. Br J Dermatol. 2000;142:899-907.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA. 1999;282:1737-1744.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Ellis GK, Robinson JA, Crawford GB. When symptoms of disease overlap with symptoms of depression. Aust Fam Physician. 2006;35:647-649.
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatry. 2013;6:151-156.
- Mattoo S, Handa S, Kaur I, et al. Psychiatric morbidity in psoriasis: prevalence and correlates in India. Ger J Psychiatry. 2005;8:17-22.
- Mattoo SK, Handa S, Kaur I, et al. Psychiatric morbidity in vitiligo and psoriasis: a comparative study from India. J Dermatol. 2001;28:424-432.
- Mehta V, Malhotra S. Psychiatric evaluation of patients with psoriasis vulgaris and chronic urticaria. Ger J Psychiatry. 2007;10:104-110.
- Meeks TW, Vahia IV, Lavretsky H, et al. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126-142.
- Hegerl U, Schönknecht P, Mergl R. Are antidepressants useful in the treatment of minor depression: a critical update of the current literature. Curr Opin Psychiatry. 2012;25:1-6.
- Rizzo M, Creed F, Goldberg D, et al. A systematic review of non-pharmacological treatments for depression in people with chronic physical health problems. J Psychosom Res. 2011;71:18-27.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9:140-147.
Psoriasis is a common immune-mediated papulosquamous skin disease with a generally chronic course. Impairments in quality of life (QOL) and psychological morbidity in the form of anxiety and depression have been reported.1 Because psoriasis is not known to directly affect the central nervous system, the associated psychiatric morbidity is likely caused by the complex interplay of the stress, physical discomfort, and possible disfigurement inherent to psoriasis, as well as the emotional response to the condition mediated by the patient’s personality, emotional and cognitive state, and other social factors (eg, self-stigma and perceived stigma, lack of knowledge about the illness in the patient and in the community and family, lack of resources and support).2 Because a variety of methodologies have been used in research on the association of psoriasis with psychiatric morbidity, it is not easy to compare findings. Most studies have assessed psychiatric symptoms rather than findings from psychiatric diagnostic instruments.3 The diagnosis of psychiatric disorders in patients with psoriasis rather than focusing on symptoms alone is likely to be more useful in generating scientific epidemiologic data and also would serve as a guide in making treatment and policy decisions. Validated clinician-rated instruments are useful in generating these data. However, psychiatric diagnoses are often missed by dermatologists, which may have an adverse impact on eventual outcomes in psoriasis patients.4,5 Patient-assessed diagnostic instruments may help dermatologists overcome this problem.
This study investigated the prevalence and determinants of psychiatric disorders in a cohort of psoriasis patients in North India using both patient self-assessment and clinician-administered instruments.
Methods
Study Participants
The study was conducted from January 2013 to November 2013 at the Postgraduate Institute of Medical Education and Research, a tertiary-level teaching hospital in Chandigarh, India, which serves the population of a large geographic area in North India. Clearance for this study was obtained from the institute ethics committee.
Patients with chronic plaque psoriasis who presented consecutively to the outpatient clinic of the Departments of Dermatology, Venereology, and Leprology during the study period were approached for participation. Written informed consent was obtained from all participants. Inclusion criteria were the ability to read the self-assessment questionnaires, and no financial compensation was offered for inclusion in the study. Patients with psoriatic arthritis as well as erythrodermic and pustular variants of psoriasis were excluded. Exclusion criteria also included patients with known diabetes mellitus, cardiovascular disease, chronic respiratory ailments, or other notable systemic comorbidities; however, patients did not undergo biochemical testing.
Assessments
A 2-stage methodology was employed. In the first stage of the assessment, sociodemographic and clinical data were recorded. Thereafter, psychiatric symptoms and morbidity were assessed using the patient health questionnaire (PHQ).6 Quality of life was assessed using the dermatology life quality index (DLQI).7 Both tools were based on patient self-assessment. Study participants could seek assistance from the clinician in completing the questionnaires, if needed. Psoriasis severity was evaluated by the clinician using the psoriasis area severity index (PASI) score.8
In the second stage of the assessment, participants underwent subsequent evaluation by a psychiatrist who was blinded to the results of the first assessments. All participants were screened using the Mini international neuropsychiatric interview (MINI)9 and a formal psychiatric diagnosis was made. In subsequent analyses, we considered psychiatric diagnoses as generated with MINI as the gold standard against which other results were compared.
A participant was considered positive for psychiatric morbidity if he/she was positive for at least 1 PHQ or MINI diagnosis.
To assess for concordance between the 2 diagnostic instruments, the following diagnostic groups were compared against each other: (1) MINI depressive disorders (DDs)(ie, major depressive episode, current and recurrent; dysthymia) versus PHQ depressive disorders (ie, major DDs and other DDs); (2) MINI anxiety disorders (ie, panic disorder and generalized anxiety disorder) versus PHQ anxiety disorders (ie, panic syndrome and other anxiety syndromes); (3) MINI alcohol abuse (ie, alcohol dependence and abuse) versus PHQ alcohol abuse; (4) comorbid disorders if more than 1 diagnosis was made; and (5) any positive score on the MINI suicide module with a response other than not at all on PHQ depression module item 2(i), which deals with thoughts of self-harm and wishing that one was dead. The MINI depressive disorders and PHQ depressive disorders indicate the presence of a clinically significant depressive state and a need for assessment and treatment.
The PHQ can be used to diagnose somatoform disorders, while the MINI cannot be used. Because the somatoform disorders diagnosed were few in number and comorbid with DDs (n=3) and anxiety disorders (n=1), we included these cases with DDs and anxiety disorders, respectively, for purposes of statistical analysis. All data were analyzed using SPSS software.
Results
One hundred four participants were included in this study. The sociodemographic, clinical, and diagnostic profiles, as well as the determinants of MINI diagnosis, are provided in Tables 1 through 4. The PASI and DLQI scores indicated that most participants had mild to moderate psoriasis severity.10 The prevalence of alcohol-related disorders was only found in the male subpopulation, which is consistent with the sociocultural context of North India. Psoriasis severity (ie, PASI score) was not found to be a determinant of psychiatric diagnoses in the study population. There was no statistical difference in measures of current clinical status and treatment modality when those with or without any psychiatric diagnoses were compared. When the variables of disease duration, treatment duration, and DLQI were entered into a binary logistic regression with positive status for a MINI diagnosis as a dependent variable indicating the presence of a psychiatric disorder, it was found that the DLQI score was a significant predictor (b=0.19; SE=0.47; χ2=17.92; P<.05). This finding was the same for regression analyses for males and females separately and also for DD as a dependent variable.
Mean DLQI and PASI scores were positively correlated with each other (Pearson r=0.23; P=.01). This relationship was maintained in males (Pearson r=0.24; P=.03) but not in females (Pearson r=0.14; P=.30). The correlations between DLQI and PASI scores and both disease duration and treatment duration were not significant. The Cohen κ values for the interrater reliability analyses done to assess the concordance of the PHQ and MINI diagnostic groups were modest (0.31-0.42), which was true even when MINI depressive disorders without dysthymia and PHQ depressive disorders were compared.
Comment
Studies investigating psychiatric morbidity in psoriasis have varying methodologies, mostly assessing psychiatric symptoms rather than screening for psychiatric disorders.3 In chronic diseases such as psoriasis, there often is an overlap between disease symptoms and common psychiatric disorders (eg, depression).11 Therefore, assessment of symptoms can be misleading. The current study was designed to detect psychiatric disorders in psoriasis patients using both patient self-assessment and clinician-administered instruments. We also investigated the contribution of sociodemographic and clinical variables (eg, psoriasis severity, impairment in QOL) on psychiatric morbidity.
An increased risk for depression, anxiety, and suicidality associated with greater psoriasis severity has been reported.12 The results of the current study indicate that even in a patient population with predominantly mild to moderate psoriasis, psychiatric morbidity, particularly DDs, is common. This finding was seen both on patient self-assessment and clinician-administered evaluations. Earlier studies from this institution and region have reported a lower prevalence of psychiatric disorders in patients with psoriasis (24.7%–36.7%).13-16 However, prior studies were based on assessment of specific symptoms and clinical diagnoses derived from history and mental status examination rather than the administration of more rigorous research diagnostic assessment tools. A systematic review and meta-analysis also revealed a lower prevalence of clinical depression using International Classification of Diseases, Tenth Revision, and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) codes.3 A possible reason for this apparent discrepancy is the fact that psychiatric morbidity in a majority of participants in the current study was constituted by the diagnosis of dysthymia. If the diagnosis of dysthymia is removed from the current analysis, the prevalence of clinical major depressive syndromes is similar to other data. We found that chronic low-grade depression (dysthymia) was the most common diagnosis from the MINI. Lower prevalence was noted in a prior study, but methodology using clinical interviewing may have resulted in an underestimated prevalence.13 It also is possible that chronic low-grade depression in psoriasis patients may be missed or underestimated in comparison to more readily diagnosed and severe depressive syndromes in other studies. However, there is enough evidence to suggest that dysthymia is clinically relevant in the causation of morbidity and disability (eg, physical, psychological, or cognitive impairment) in patients with chronic physical disorders.17 This distinction between clinical major depressive syndrome and dysthymia is important because different treatment methods may be required; the former may warrant treatment with antidepressants in addition to psychosocial treatment modalities (eg, learning to cope with stress, problem-solving techniques), while the latter may benefit predominantly from psychosocial treatment modalities alone.18,19 In the current study, most of the participants who were diagnosed with dysthymia refused treatment with any psychotropic medications but perceived benefit from discussing their problems with a professional. Our results indicated that chronic low-grade depression is more common than more severe major depressive states, and mental health professionals who are well versed in psychosocial treatment modalities should play an integral role in treatment planning for patients with psoriasis.
In the current study, there was only a modest correlation between the results of the patient self-assessment and clinician-administered evaluations, which indicated that psychiatric disorders may not be obvious to clinicians unless specifically investigated, even for some severely depressed and suicidal patients. Given the high prevalence of clinically relevant psychiatric morbidity among psoriasis patients, dermatology professionals should be more sensitive to the possible presence of psychiatric disorders in this patient population and should consider the use of formal screening or other diagnostic tools for detection of depression and anxiety in psoriasis patients.
The main determinant of psychiatric morbidity in our study population was impairment in QOL. Interestingly enough, psoriasis severity was not associated with psychiatric morbidity in our study. Depressive states in patients with chronic physical illnesses are well known and could be due to the chronic stress of illness or impaired QOL, or depression may be a direct effect of the illness and/or treatment on the central nervous system. Psoriasis is not known to have any direct effect on the central nervous system. Our findings suggest that QOL impairment plays an important role in psychiatric morbidity in patients with psoriasis. Even though the DLQI is designed to measure QOL over the preceding week, our findings suggest that impairment in QOL in psoriasis is a manifestation of a more long-term effect of interplay between many factors; the impairment in activities of daily living, disease-related physical discomfort and impaired self-esteem and self-perception, impairments in interpersonal relationships, and the stress of chronicity of illness seem to play an important role. Additionally, variables such as emotional dysfunction, magnitude and site of the area of involvement, nature and magnitude of comorbidities, and complications of illness and coping also may be relevant.20 Although these factors are common in other chronic disorders, psoriasis in particular may predisposepatients to depression due to its unpredictable and relapsing nature, lack of any curative therapy, and the stigmatizing prominent lesions that often are impossible to camouflage. In chronic diseases such as psoriasis, the amelioration of impairment of different aspects of QOL may be more important than mere symptom control.
Our study was limited in that the study population was predominantly male. Fewer females may have consented to participate in the study due to time constraints associated with domestic responsibilities, reluctance to discuss psychological distress, or inability to meet the inclusion criteria (eg, level of education required to read questionnaires). However, there was no significant difference between males and females for sociodemographic variables or diagnoses other than alcohol-related disorders. Our study also had a cross-sectional design and there was no control group, without which it is difficult to assess the true prevalence and determinants of these psychiatric morbidities. Moreover, the sample size was small and did not include enough participants with moderate to severe psoriasis (ie, PASI score ≥10) to be able to detect a correlation between psychiatric morbidities and psoriasis severity. Our findings underline the need for effective screening and integrated management of psychiatric disorders in patients with psoriasis.
Psoriasis is a common immune-mediated papulosquamous skin disease with a generally chronic course. Impairments in quality of life (QOL) and psychological morbidity in the form of anxiety and depression have been reported.1 Because psoriasis is not known to directly affect the central nervous system, the associated psychiatric morbidity is likely caused by the complex interplay of the stress, physical discomfort, and possible disfigurement inherent to psoriasis, as well as the emotional response to the condition mediated by the patient’s personality, emotional and cognitive state, and other social factors (eg, self-stigma and perceived stigma, lack of knowledge about the illness in the patient and in the community and family, lack of resources and support).2 Because a variety of methodologies have been used in research on the association of psoriasis with psychiatric morbidity, it is not easy to compare findings. Most studies have assessed psychiatric symptoms rather than findings from psychiatric diagnostic instruments.3 The diagnosis of psychiatric disorders in patients with psoriasis rather than focusing on symptoms alone is likely to be more useful in generating scientific epidemiologic data and also would serve as a guide in making treatment and policy decisions. Validated clinician-rated instruments are useful in generating these data. However, psychiatric diagnoses are often missed by dermatologists, which may have an adverse impact on eventual outcomes in psoriasis patients.4,5 Patient-assessed diagnostic instruments may help dermatologists overcome this problem.
This study investigated the prevalence and determinants of psychiatric disorders in a cohort of psoriasis patients in North India using both patient self-assessment and clinician-administered instruments.
Methods
Study Participants
The study was conducted from January 2013 to November 2013 at the Postgraduate Institute of Medical Education and Research, a tertiary-level teaching hospital in Chandigarh, India, which serves the population of a large geographic area in North India. Clearance for this study was obtained from the institute ethics committee.
Patients with chronic plaque psoriasis who presented consecutively to the outpatient clinic of the Departments of Dermatology, Venereology, and Leprology during the study period were approached for participation. Written informed consent was obtained from all participants. Inclusion criteria were the ability to read the self-assessment questionnaires, and no financial compensation was offered for inclusion in the study. Patients with psoriatic arthritis as well as erythrodermic and pustular variants of psoriasis were excluded. Exclusion criteria also included patients with known diabetes mellitus, cardiovascular disease, chronic respiratory ailments, or other notable systemic comorbidities; however, patients did not undergo biochemical testing.
Assessments
A 2-stage methodology was employed. In the first stage of the assessment, sociodemographic and clinical data were recorded. Thereafter, psychiatric symptoms and morbidity were assessed using the patient health questionnaire (PHQ).6 Quality of life was assessed using the dermatology life quality index (DLQI).7 Both tools were based on patient self-assessment. Study participants could seek assistance from the clinician in completing the questionnaires, if needed. Psoriasis severity was evaluated by the clinician using the psoriasis area severity index (PASI) score.8
In the second stage of the assessment, participants underwent subsequent evaluation by a psychiatrist who was blinded to the results of the first assessments. All participants were screened using the Mini international neuropsychiatric interview (MINI)9 and a formal psychiatric diagnosis was made. In subsequent analyses, we considered psychiatric diagnoses as generated with MINI as the gold standard against which other results were compared.
A participant was considered positive for psychiatric morbidity if he/she was positive for at least 1 PHQ or MINI diagnosis.
To assess for concordance between the 2 diagnostic instruments, the following diagnostic groups were compared against each other: (1) MINI depressive disorders (DDs)(ie, major depressive episode, current and recurrent; dysthymia) versus PHQ depressive disorders (ie, major DDs and other DDs); (2) MINI anxiety disorders (ie, panic disorder and generalized anxiety disorder) versus PHQ anxiety disorders (ie, panic syndrome and other anxiety syndromes); (3) MINI alcohol abuse (ie, alcohol dependence and abuse) versus PHQ alcohol abuse; (4) comorbid disorders if more than 1 diagnosis was made; and (5) any positive score on the MINI suicide module with a response other than not at all on PHQ depression module item 2(i), which deals with thoughts of self-harm and wishing that one was dead. The MINI depressive disorders and PHQ depressive disorders indicate the presence of a clinically significant depressive state and a need for assessment and treatment.
The PHQ can be used to diagnose somatoform disorders, while the MINI cannot be used. Because the somatoform disorders diagnosed were few in number and comorbid with DDs (n=3) and anxiety disorders (n=1), we included these cases with DDs and anxiety disorders, respectively, for purposes of statistical analysis. All data were analyzed using SPSS software.
Results
One hundred four participants were included in this study. The sociodemographic, clinical, and diagnostic profiles, as well as the determinants of MINI diagnosis, are provided in Tables 1 through 4. The PASI and DLQI scores indicated that most participants had mild to moderate psoriasis severity.10 The prevalence of alcohol-related disorders was only found in the male subpopulation, which is consistent with the sociocultural context of North India. Psoriasis severity (ie, PASI score) was not found to be a determinant of psychiatric diagnoses in the study population. There was no statistical difference in measures of current clinical status and treatment modality when those with or without any psychiatric diagnoses were compared. When the variables of disease duration, treatment duration, and DLQI were entered into a binary logistic regression with positive status for a MINI diagnosis as a dependent variable indicating the presence of a psychiatric disorder, it was found that the DLQI score was a significant predictor (b=0.19; SE=0.47; χ2=17.92; P<.05). This finding was the same for regression analyses for males and females separately and also for DD as a dependent variable.
Mean DLQI and PASI scores were positively correlated with each other (Pearson r=0.23; P=.01). This relationship was maintained in males (Pearson r=0.24; P=.03) but not in females (Pearson r=0.14; P=.30). The correlations between DLQI and PASI scores and both disease duration and treatment duration were not significant. The Cohen κ values for the interrater reliability analyses done to assess the concordance of the PHQ and MINI diagnostic groups were modest (0.31-0.42), which was true even when MINI depressive disorders without dysthymia and PHQ depressive disorders were compared.
Comment
Studies investigating psychiatric morbidity in psoriasis have varying methodologies, mostly assessing psychiatric symptoms rather than screening for psychiatric disorders.3 In chronic diseases such as psoriasis, there often is an overlap between disease symptoms and common psychiatric disorders (eg, depression).11 Therefore, assessment of symptoms can be misleading. The current study was designed to detect psychiatric disorders in psoriasis patients using both patient self-assessment and clinician-administered instruments. We also investigated the contribution of sociodemographic and clinical variables (eg, psoriasis severity, impairment in QOL) on psychiatric morbidity.
An increased risk for depression, anxiety, and suicidality associated with greater psoriasis severity has been reported.12 The results of the current study indicate that even in a patient population with predominantly mild to moderate psoriasis, psychiatric morbidity, particularly DDs, is common. This finding was seen both on patient self-assessment and clinician-administered evaluations. Earlier studies from this institution and region have reported a lower prevalence of psychiatric disorders in patients with psoriasis (24.7%–36.7%).13-16 However, prior studies were based on assessment of specific symptoms and clinical diagnoses derived from history and mental status examination rather than the administration of more rigorous research diagnostic assessment tools. A systematic review and meta-analysis also revealed a lower prevalence of clinical depression using International Classification of Diseases, Tenth Revision, and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) codes.3 A possible reason for this apparent discrepancy is the fact that psychiatric morbidity in a majority of participants in the current study was constituted by the diagnosis of dysthymia. If the diagnosis of dysthymia is removed from the current analysis, the prevalence of clinical major depressive syndromes is similar to other data. We found that chronic low-grade depression (dysthymia) was the most common diagnosis from the MINI. Lower prevalence was noted in a prior study, but methodology using clinical interviewing may have resulted in an underestimated prevalence.13 It also is possible that chronic low-grade depression in psoriasis patients may be missed or underestimated in comparison to more readily diagnosed and severe depressive syndromes in other studies. However, there is enough evidence to suggest that dysthymia is clinically relevant in the causation of morbidity and disability (eg, physical, psychological, or cognitive impairment) in patients with chronic physical disorders.17 This distinction between clinical major depressive syndrome and dysthymia is important because different treatment methods may be required; the former may warrant treatment with antidepressants in addition to psychosocial treatment modalities (eg, learning to cope with stress, problem-solving techniques), while the latter may benefit predominantly from psychosocial treatment modalities alone.18,19 In the current study, most of the participants who were diagnosed with dysthymia refused treatment with any psychotropic medications but perceived benefit from discussing their problems with a professional. Our results indicated that chronic low-grade depression is more common than more severe major depressive states, and mental health professionals who are well versed in psychosocial treatment modalities should play an integral role in treatment planning for patients with psoriasis.
In the current study, there was only a modest correlation between the results of the patient self-assessment and clinician-administered evaluations, which indicated that psychiatric disorders may not be obvious to clinicians unless specifically investigated, even for some severely depressed and suicidal patients. Given the high prevalence of clinically relevant psychiatric morbidity among psoriasis patients, dermatology professionals should be more sensitive to the possible presence of psychiatric disorders in this patient population and should consider the use of formal screening or other diagnostic tools for detection of depression and anxiety in psoriasis patients.
The main determinant of psychiatric morbidity in our study population was impairment in QOL. Interestingly enough, psoriasis severity was not associated with psychiatric morbidity in our study. Depressive states in patients with chronic physical illnesses are well known and could be due to the chronic stress of illness or impaired QOL, or depression may be a direct effect of the illness and/or treatment on the central nervous system. Psoriasis is not known to have any direct effect on the central nervous system. Our findings suggest that QOL impairment plays an important role in psychiatric morbidity in patients with psoriasis. Even though the DLQI is designed to measure QOL over the preceding week, our findings suggest that impairment in QOL in psoriasis is a manifestation of a more long-term effect of interplay between many factors; the impairment in activities of daily living, disease-related physical discomfort and impaired self-esteem and self-perception, impairments in interpersonal relationships, and the stress of chronicity of illness seem to play an important role. Additionally, variables such as emotional dysfunction, magnitude and site of the area of involvement, nature and magnitude of comorbidities, and complications of illness and coping also may be relevant.20 Although these factors are common in other chronic disorders, psoriasis in particular may predisposepatients to depression due to its unpredictable and relapsing nature, lack of any curative therapy, and the stigmatizing prominent lesions that often are impossible to camouflage. In chronic diseases such as psoriasis, the amelioration of impairment of different aspects of QOL may be more important than mere symptom control.
Our study was limited in that the study population was predominantly male. Fewer females may have consented to participate in the study due to time constraints associated with domestic responsibilities, reluctance to discuss psychological distress, or inability to meet the inclusion criteria (eg, level of education required to read questionnaires). However, there was no significant difference between males and females for sociodemographic variables or diagnoses other than alcohol-related disorders. Our study also had a cross-sectional design and there was no control group, without which it is difficult to assess the true prevalence and determinants of these psychiatric morbidities. Moreover, the sample size was small and did not include enough participants with moderate to severe psoriasis (ie, PASI score ≥10) to be able to detect a correlation between psychiatric morbidities and psoriasis severity. Our findings underline the need for effective screening and integrated management of psychiatric disorders in patients with psoriasis.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Richards HL, Fortune DG, Weidmann A, et al. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol. 2004;151:1227-1233.
- Scharloo M, Kaptein AA, Weinman J, et al. Patients’ illness perceptions and coping as predictors of functional status in psoriasis: a 1-year follow-up. Br J Dermatol. 2000;142:899-907.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA. 1999;282:1737-1744.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Ellis GK, Robinson JA, Crawford GB. When symptoms of disease overlap with symptoms of depression. Aust Fam Physician. 2006;35:647-649.
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatry. 2013;6:151-156.
- Mattoo S, Handa S, Kaur I, et al. Psychiatric morbidity in psoriasis: prevalence and correlates in India. Ger J Psychiatry. 2005;8:17-22.
- Mattoo SK, Handa S, Kaur I, et al. Psychiatric morbidity in vitiligo and psoriasis: a comparative study from India. J Dermatol. 2001;28:424-432.
- Mehta V, Malhotra S. Psychiatric evaluation of patients with psoriasis vulgaris and chronic urticaria. Ger J Psychiatry. 2007;10:104-110.
- Meeks TW, Vahia IV, Lavretsky H, et al. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126-142.
- Hegerl U, Schönknecht P, Mergl R. Are antidepressants useful in the treatment of minor depression: a critical update of the current literature. Curr Opin Psychiatry. 2012;25:1-6.
- Rizzo M, Creed F, Goldberg D, et al. A systematic review of non-pharmacological treatments for depression in people with chronic physical health problems. J Psychosom Res. 2011;71:18-27.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9:140-147.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Richards HL, Fortune DG, Weidmann A, et al. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol. 2004;151:1227-1233.
- Scharloo M, Kaptein AA, Weinman J, et al. Patients’ illness perceptions and coping as predictors of functional status in psoriasis: a 1-year follow-up. Br J Dermatol. 2000;142:899-907.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA. 1999;282:1737-1744.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Ellis GK, Robinson JA, Crawford GB. When symptoms of disease overlap with symptoms of depression. Aust Fam Physician. 2006;35:647-649.
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatry. 2013;6:151-156.
- Mattoo S, Handa S, Kaur I, et al. Psychiatric morbidity in psoriasis: prevalence and correlates in India. Ger J Psychiatry. 2005;8:17-22.
- Mattoo SK, Handa S, Kaur I, et al. Psychiatric morbidity in vitiligo and psoriasis: a comparative study from India. J Dermatol. 2001;28:424-432.
- Mehta V, Malhotra S. Psychiatric evaluation of patients with psoriasis vulgaris and chronic urticaria. Ger J Psychiatry. 2007;10:104-110.
- Meeks TW, Vahia IV, Lavretsky H, et al. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126-142.
- Hegerl U, Schönknecht P, Mergl R. Are antidepressants useful in the treatment of minor depression: a critical update of the current literature. Curr Opin Psychiatry. 2012;25:1-6.
- Rizzo M, Creed F, Goldberg D, et al. A systematic review of non-pharmacological treatments for depression in people with chronic physical health problems. J Psychosom Res. 2011;71:18-27.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9:140-147.
Practice Points
- Psychiatric disorders, especially depressive disorders, are common in patients with psoriasis.
- Impairments in quality of life in patients with psoriasis can predict psychiatric morbidity.
- Screening for psychiatric disorders and depression in particular should be considered in patients with psoriasis.
- Treatment should focus not just on symptom alleviation but also coping with the effects of living with a chronic illness such as psoriasis and improving quality of life.