User login
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
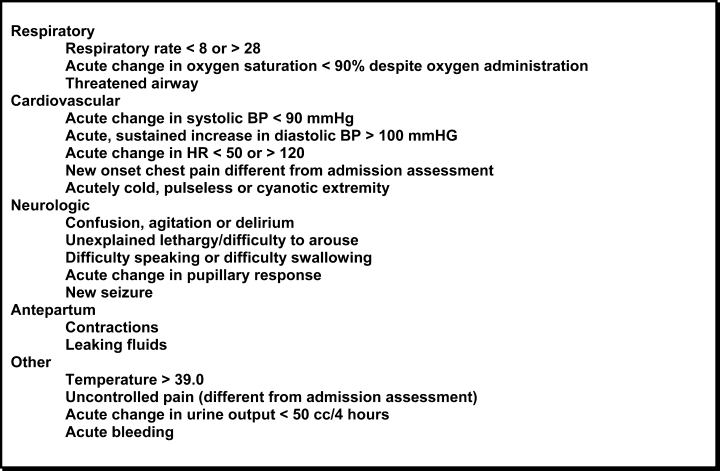
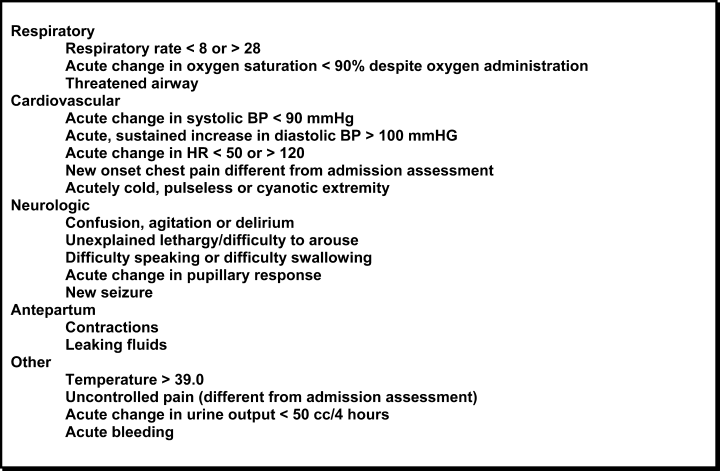
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.
Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.