User login
The Diagnosis: Lymphomatoid Papulosis
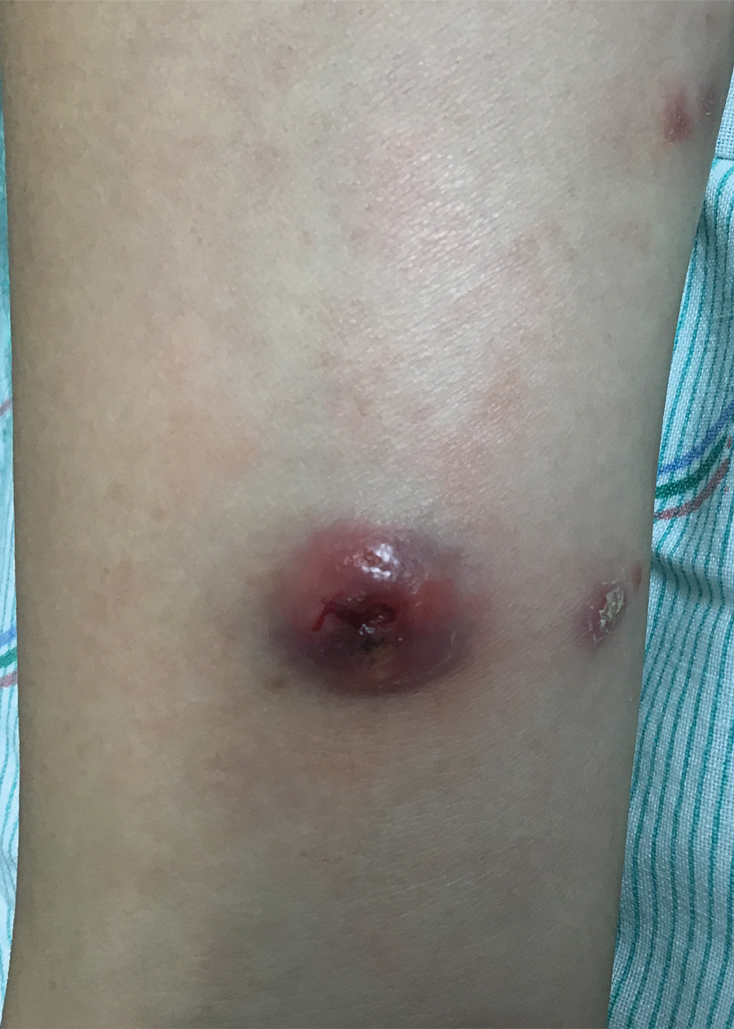
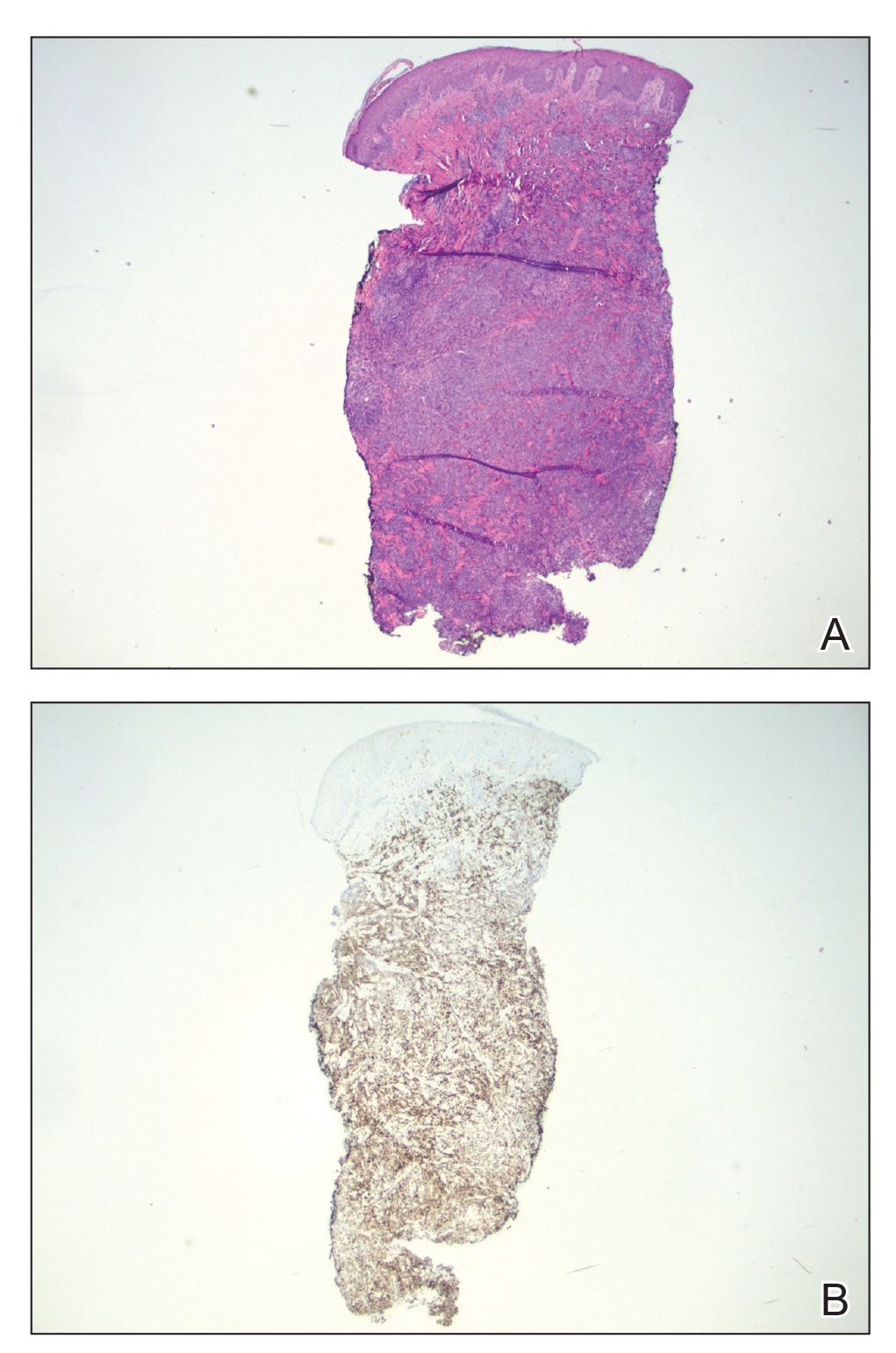
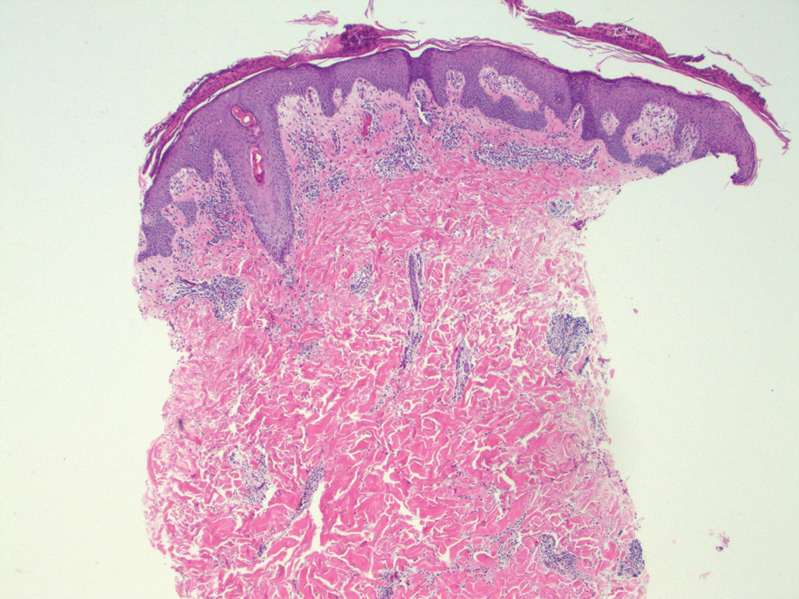
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.
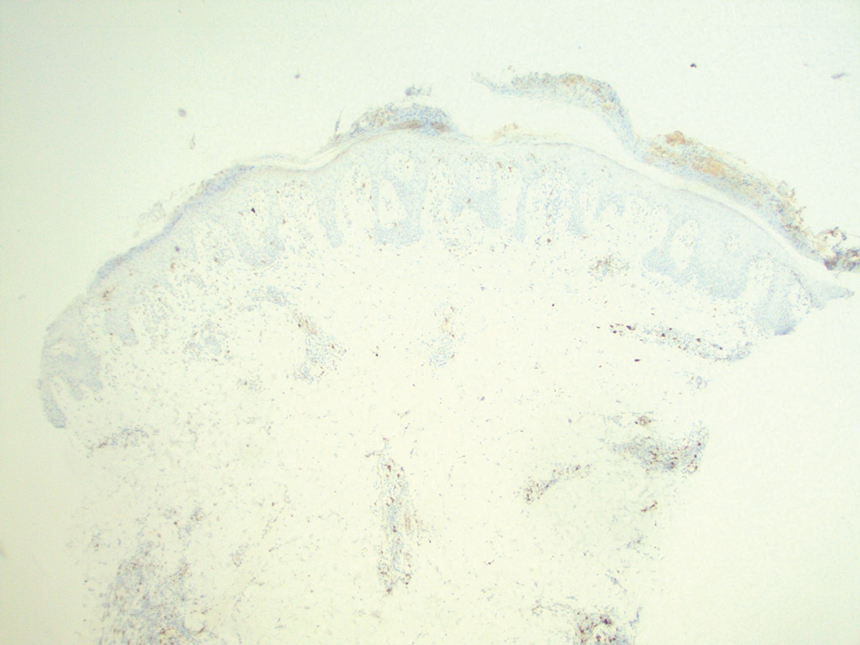
Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
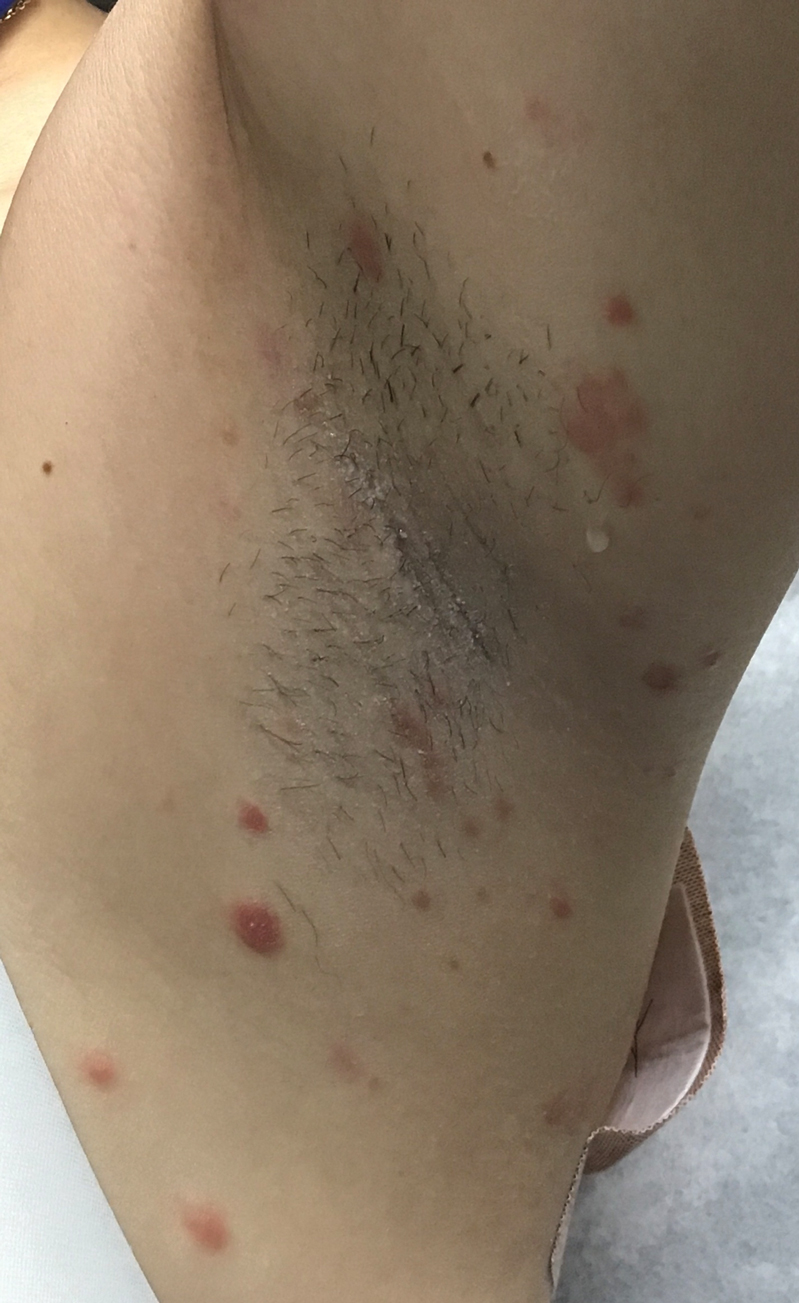
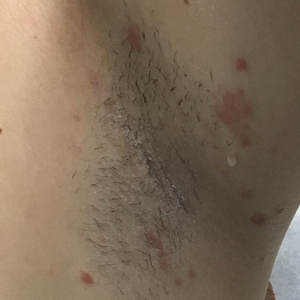
A 37-year-old woman presented to our dermatology clinic with a pruritic erythematous eruption involving the trunk, axillae, and proximal extremities of 10 days’ duration. Her medical history was notable only for eczema, and she denied taking any medications. Physical examination revealed scattered erythematous papules and crusts involving the trunk bilaterally and the extremities. We initially made a clinical diagnosis of bullous impetigo, and the patient was prescribed mupirocin ointment and dicloxacillin. At 1-week follow-up, the patient reported persistent skin lesions that were evolving despite therapy. Physical examination at this visit revealed an evolving eruption of multiple reddish-brown scaly papules involving the axillae, arms, forearms, and thighs, as depicted here.