User login
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
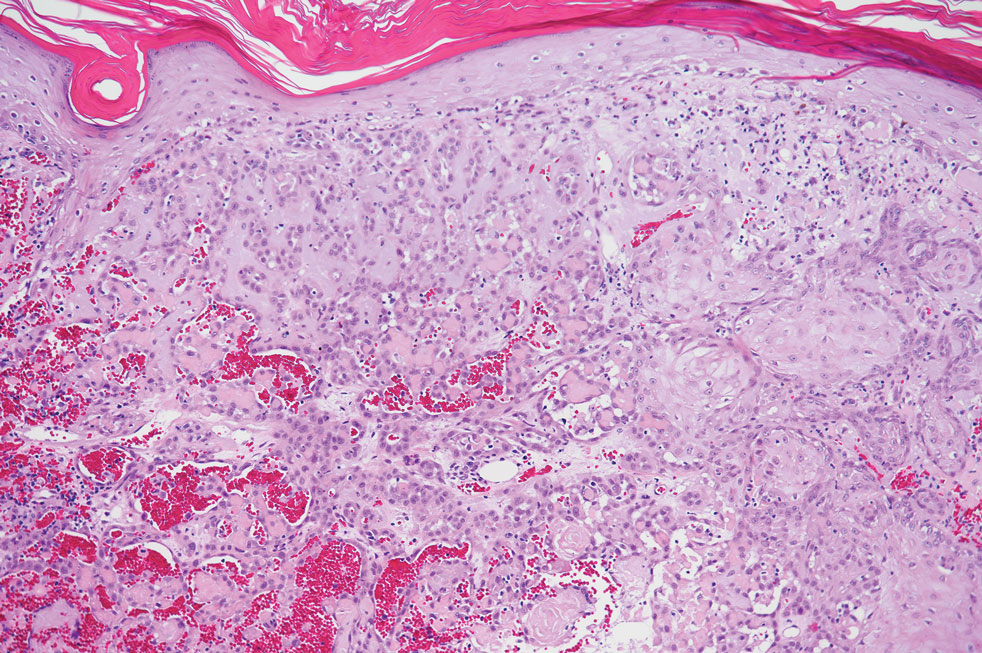
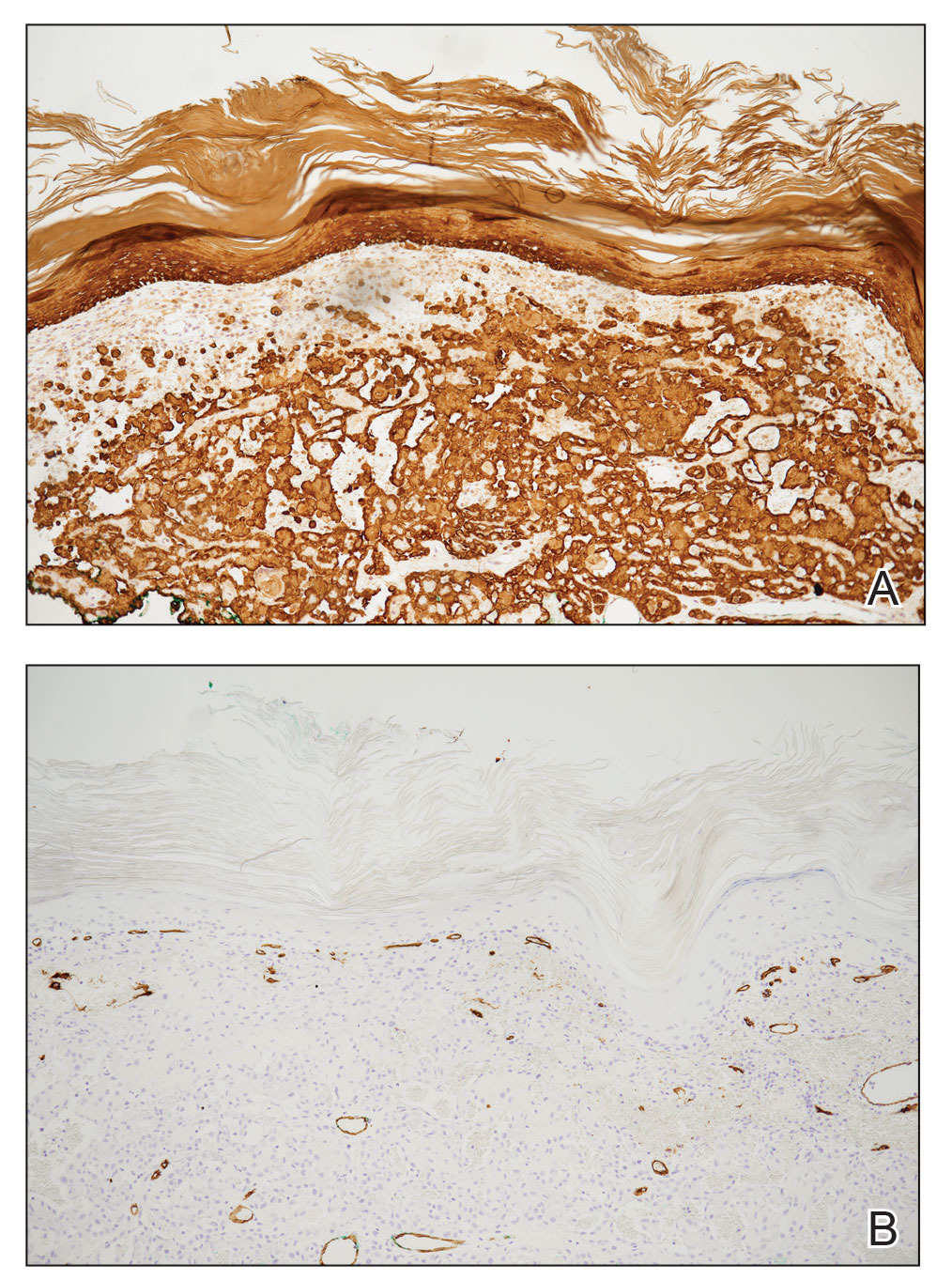
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.
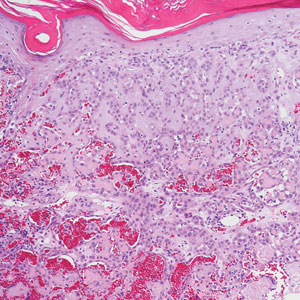
Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.
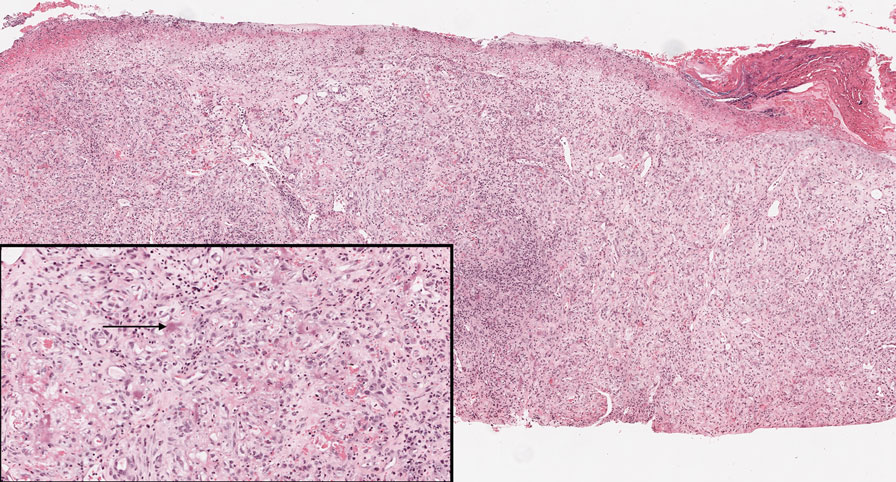
Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.
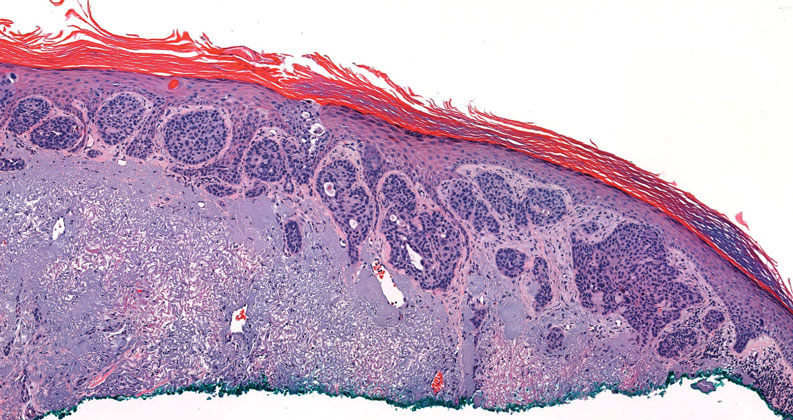
Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.
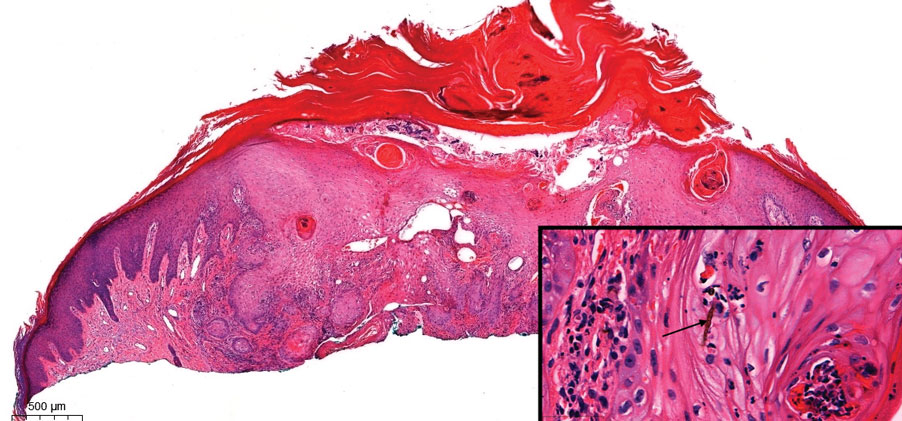
Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
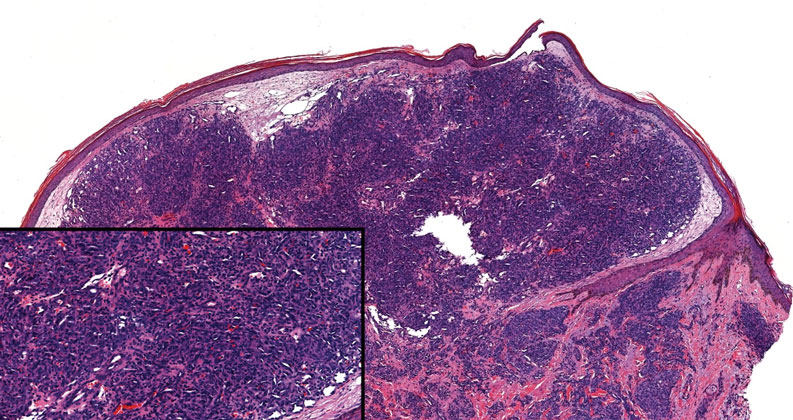
An 84-year-old man with a history of nonmelanoma skin cancer presented to our clinic with a 1.6×1.5-cm exophytic lesion on the left posterior parietal scalp. The lesion nearly doubled in size over the last 4 months. The patient received radiation therapy in this area for the treatment of basal cell carcinoma 7 years prior to presentation. A shave biopsy was performed.