User login
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
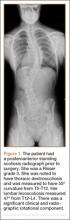
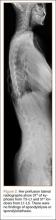
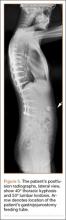
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
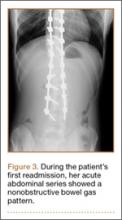
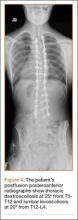
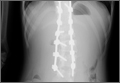
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
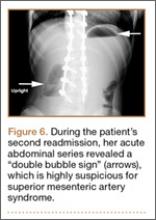
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
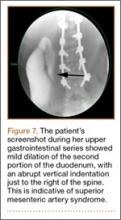
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
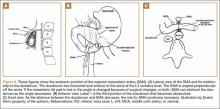
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.