User login
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
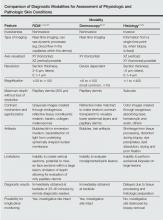
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
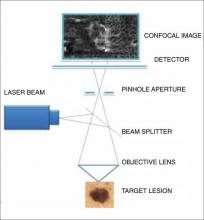
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
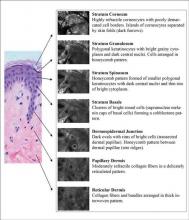
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
Reflectance confocal microscopy (RCM) creates an image by detecting backscattered light from illuminated tissue and displaying it on a monitor at high resolution and contrast. The grayscale images, oriented in a horizontal (en face) plane, reveal cellular and morphologic architecture in progressive depths from the epidermis to the papillary dermis.1,2 Analyses of confocal features have shown good correlation with histologic and dermoscopic findings, allowing key features of normal skin topography as well as cutaneous lesions to be delineated.1-15 Most research has focused on differentiating benign and malignant lesions, but RCM also has proven utility in presurgical mapping and in monitoring therapeutic efficacy of topical treatments of malignancies.16-19 Most recently, this US Food and Drug Administration 510(k)-cleared tool for imaging in vivo unstained epithelium (including blood, collagen, and pigment) has an added adjunct: a telepathology network dedicated to the transfer of confocal images from a private practice to a remote confocal diagnostic reader for image interpretation. As a noninvasive technique, RCM is a promising tool, not only in the field of dermatology but also in primary care.
Comparison of Diagnostic Modalities
Historically, diagnostic modalities have included visual and histopathologic examination; however, basing a diagnosis solely on clinical grounds may not be reliable, and obtaining a tissue specimen may not be feasible or practical. Thus, noninvasive modalities as adjuncts for evaluation were developed, including high-frequency ultrasound, high-definition optical coherence tomography, dermoscopy, and in vivo RCM. Sonography was not reliable in clinical practice due to poor echogenicity and insufficient resolution, and although high-definition optical coherence tomography is emerging as an important tool for the evaluation of lesions with high clinical suspicion for nonmelanoma skin cancers (eg, basal cell carcinoma [BCC]), resolution is still insufficient for definitive diagnosis; therefore, these devices cannot be reliably used on pigmented lesions suspicious for melanoma.20-23
Reflectance confocal microscopy has many properties similar to both dermoscopy and histology (Table).1-3,24-28 Dermoscopy and RCM are used by physicians to noninvasively analyze lesions en face in real time; both modalities operate through optical magnification and liquid immersion without exogenous contrast agents and can be used to monitor lesion progression over time.25,29 However, when comparing these modalities for melanoma identification among equivocal melanocytic lesions, they revealed statistically similar sensitivities (dermoscopy, 88%; RCM, 91%) but notably different specificities with RCM achieving more than double the specificity (dermoscopy, 32%; RCM, 68%).30
Similar to histology images, RCM images provide high axial and lateral resolution, delineating cellular and morphologic architecture in both vertical and horizontal planes.29,31 Unlike histology, RCM does not require tissue removal and processing, thus the images are immediately available for analysis. Although RCM is noninvasive similar to dermoscopy and has resolutions comparable to histology, it uniquely demonstrates the dynamic processes of living skin in real time.1-3
Technical Properties of RCM
There are 7 components to the microscope: a laser light source, scanning elements, a relay telescope, a beam splitter, a pinhole aperture, an objective lens, and a detector (Figure 1).1,2,32 A low-power laser beam illuminates a point on or within the skin. The scattered light reflected back into the optical system is imaged on a detector. A pinhole aperture in front of this detector filters out the scattered light and allows only the light from the image plane (a thin, in-focus plane in the tissue) to pass through, creating a high-resolution image (3–5 μm horizontal optical sections) of the target lesion. The optical parameters include an 830-nm laser with an operating power of 22 mW and an immersion objective lens with a 0.9 numerical aperture. Each image has a 500-μm field of view with approximately ×30 magnification. A larger 2-dimensional image is created when the laser rapidly scans across the plane of the skin lesion, sequentially capturing multiple images. These individual images are stitched together to create a mosaic with a field of up to 8×8 mm.1,2,32
The maximum imaging depth extends into the papillary dermis (up to 350 mm, depending on tissue thickness).1,2,27,32 Depth of light penetration is limited by wavelength and intensity to maximize resolution of discernible structures and to avoid tissue damage. Contrast is dependent on light scattering, which is generated in 2 ways: (1) by differences between the refractive index of water and tissue constituents, and (2) by diffraction of light by structures similar in size to the illuminating light wavelength. Thus, highly reflective or diffractive structures such as melanin, keratin, hydrated collagen, and melanosomes produce backscattered light that appears bright (white) compared to their surroundings.1,2,27,32
RCM Image Acquisition and Interpretation
After patient and lesion history are obtained, visual and dermoscopic evaluation of the lesion is performed. Index fluid (mineral oil) is applied to the lesion and a metal ring with an optically clear, nonbirefringent polymer window is attached to the skin.32 A 5-megapixel dermoscopic-quality image is captured through this ring and window. A water-based immersion medium (ultrasound gel) for the objective lens is then placed on the window and the confocal microscope is magnetically attached to the ring. The index fluid has a refractive index similar to the stratum corneum and the window, allowing for optimal imaging down to the papillary dermis. The adhesive and magnetic attachments stabilize the skin lesion as the confocal microscope captures sequential 2-dimensional images. A mosaic is then created at the specified anatomic level. Levels of imaging are determined by the depth (in micrometers) from the stratum corneum and correspond to each anatomic layer. En face images also can be vertically stacked, generally in 3- to 5-μm increments.32
The number of mosaics and stacks obtained are based on a preset standardized protocol. Once captured, images are saved and then sent over the telemedicine network to a remote confocal diagnostic reader for image interpretation, which can be done immediately or the images can be stored and sent (known as store and forward) at a later time.
Lesion evaluation begins with a review of patient and lesion history and dermoscopic images, followed by review of confocal images for additional information through visualization of cellular structures and architecture. Confocal interpretation commonly begins with examination of the mosaic at the level of the dermoepidermal junction, as it often provides the most diagnostic information. The papillary epidermal layers can then be used to confirm the working diagnosis, to further enhance the description, or to refine the diagnosis. Areas of interest may then be further examined in a vertical plane, which is achieved by observing a specific spot at different depths. Image interpretation can be approached in a variety of ways; however, the most critical aspect to any method is the recognition of skin morphology.
Confocal Images
Epidermal and dermal structures identified with in vivo confocal images are comparable to histology. The first consensus terminology glossary with illustrative images was published in 2007 with descriptions and definitions of image quality, normal skin morphology, lesional architecture, and cellular details of melanocytic lesions.33 Figure 2 shows the normal structures that comprise the different layers of skin as seen on RCM.
Stratum Corneum
At a thickness of 0 to 15 μm, the stratum corneum is the first bright image seen on RCM.1,2 The individual anucleate corneocytes often cannot be delineated; thus, sheets of cells appear as islands separated by dark furrows (wrinkles).1,2
Stratum Granulosum
The first layer of viable cells is located 15 to 20 μm below the skin’s surface.1,2 The large 25- to 35-μm polygonal structures (granular keratinocytes) contain bright border zones (cytoplasm), a central dark oval (nucleus), and a grainy appearance (keratohyalin granules, organelles, and melanosomes).1-3 A honeycomb pattern is seen within the normal epidermis, whereas in darkly pigmented lesions where keratinocytes may contain some pigment, it has been described as cobblestone pattern.3
Stratum Spinosum
At a depth of 20 to 50 μm, the honeycomb pattern consists of smaller 15- to 25-μm polygonal structures (spinous keratinocytes) with thinner bright borders (cytoplasm) and a darker oval nucleus.1-3
Stratum Basale
Below the spinous cells is a single layer of brighter round structures (basal keratinocytes), each 7 to 12 mm in diameter.1,2 Due to the supranuclear melanin caps, the basal keratinocytes have increased reflectivity, appearing brighter than granular and spinous cells. The more abundant the melanin within the basal keratinocytes, the brighter the appearance.1,2
Dermoepidermal Junction
Below the stratum corneum (50–150 μm) is the dermoepidermal junction.1 The “peaks” of dermal papillae emerge as clusters of bright cells (basal keratinocytes). With deeper sectioning, the dark round-oval spaces rimmed by bright basal cells (dermal papillae) progressively enlarge. They continue to enlarge until neighboring papillae touch each other tangentially, corresponding to the valleys of rete ridges.1
Papillary Dermis
At a depth of 60 to 80 μm, blood vessels and collagen fibers are seen.1-3 Collagen and elastin fibers present as thin, delicately intertwined, highly reflective fibrillar structures (1–5 μm). Blood vessels appear as weakly reflective, round or canalicular structures within dermal papillae. Within the lumina, serum appears dark, but blood cells can be seen in real time as continually moving, weakly reflective or bright round structures corresponding to leukocytes, erythrocytes, and platelets. With real-time imaging, cells also can be identified based on their movement; leukocytes can fill or distend the lumen and roll slowly along vessel walls, whereas erythrocytes move rapidly within vessel lumina.1-3
Reticular Dermis
Further below the stratum corneum (100–350 μm), similar highly reflective collagen fibers and bundles are present, with diameters of 1 to 5 μm and 5 to 25 μm, respectively.2,3
Adnexal Structures
The limitation of imaging depth by wavelength and intensity restricts visualization to upper portions of sebaceous glands, sweat ducts, and hair shafts within hair follicles.2,32
Clinical Applications of RCM
Diagnosis of Lesions
Since the inception of RCM, confocal-based diagnostic criteria have been established for allergic and irritant contact dermatitis,4,5 malignant melanoma,6 BCC,7 actinic keratosis,8 and squamous cell carcinoma.8 Much of the research has focused on skin cancers, including the differentiation of benign and malignant skin lesions,34-38 to help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.10,11,28,35,38 In 2008 Guitera et al39 used RCM and dermoscopy to detect melanoma with a sensitivity of 98% and in 2012 determined that biopsies of benign nevi and lesions clinically suspicious for BCC could be reduced by as much as 68% in a series of 710 equivocal lesions.35 In 2014, in a prospective study including more than 1000 patients, Pellacani et al38 demonstrated that biopsies of equivocal benign lesions were reduced by more that 50%, and all of the melanomas and BCCs excised in the study were correctly detected by RCM interpretation. Additionally, in both studies, the sensitivity of the RCM interpretation for detecting BCC was 100%. Amelanotic melanoma can be diagnostically challenging because clinical and dermoscopic features often are nondescript. In 2001, Busam et al17 successfully used RCM for amelanotic melanoma detection and margin assessment. A subsequent study by Braga et al24 positively demonstrated that RCM may aid in the detection and diagnosis of various solitary pink lesions.
Adjunct to Mohs Micrographic Surgery
When excisional biopsies are impractical, incisional biopsies may be performed, which may lead to sampling errors. Atypical lesions with poorly defined clinical borders dictates standard of care with surgical excision and microscopic evaluation of margins. For malignancies requiring treatment with Mohs micrographic surgery, further staging often is required. These limitations may be overcome with RCM. Early detection of amelanotic malignant melanoma with margin assessment has been successfully demonstrated.17 Curiel-Lewandroski et al16 reported 3 successful cases wherein RCM was used for diagnosis and monitoring of topical treatment, delineation of surgical margins, and guidance in tissue-sparing surgical excision with amelanotic melanoma, locally recurrent melanoma, and lentigo maligna melanoma, respectively. In 2013, Guitera et al40 demonstrated that mapping lentigo maligna margins prior to Mohs surgery changed the surgical management of 73% of patients in a study that included 37 patients with clinically or dermoscopically visible lesions.
Monitoring Topical Treatment
Unlike conventional histology, RCM does not involve tissue destruction, allowing for longitudinal surveillance when treating a malignancy with topical therapy. In a 2003 case study, RCM was used to confirm a previously diagnosed BCC, map tumor periphery, visualize the inflammatory response to imiquimod cream 5%, and confirm posttreatment clearance. Reflectance confocal microscopy features were confirmed with biopsy before and after treatment, and clinical findings during treatment precisely correlated with RCM findings.18 A similar study the following year demonstrated the efficacy of imiquimod cream 5% as an adjunct to BCC treatment by reducing or eliminating the lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.19 To date, several studies have been performed by physicians throughout the world that have used RCM to monitor therapeutic outcomes of topically applied treatments such as imiquimod and hyaluronic acid as well as photodynamic therapy.41-43
A Clinical Tool
In vivo reflectance confocal microscopy, previously used only in the research setting, is now being used as a clinical tool for the evaluation of lesions suspicious for skin cancer by several academic centers and private practices throughout the United States. With clearance from the US Food and Drug Administration, physicians can use the device clinically for in vivo microscopic examination of skin lesions. The telepathology network allows for images to be acquired by a trained technician in a clinician’s office and then to be evaluated remotely by a diagnostic reader. The clinician can receive a diagnosis in as little as 30 minutes. The potential to noninvasively monitor tumor response to topical therapies, to delineate tumor margins prior to surgery, and to monitor lesions over time is an attractive option to patients.
The technology and telepathology network of RCM continues to be developed as diagnostic criteria are established and diagnostic readers are trained; however, diagnostic confocal features of various lesions have yet to be described, refined, or validated. Consequently, an extensive library of reference images has not yet been constructed.
Practical Application
A dermatology practice collaborated with a dermatopathology office to examine the feasibility of incorporating RCM and the telepathology network into the workflow of a private practice while creating a comprehensive library of cutaneous pathologies. A physician who did not have prior knowledge of RCM was selected for training with the goal to become proficient at operating the confocal microscope and interpreting the images. A dermatopathologist (also a confocal diagnostic reader) performed the histopathologic diagnoses of the lesions and correlated findings to confocal images.
Once images were captured using a standardized protocol, the lesion was biopsied according to standard of care. The images were sent over the telepathology network for interpretation and correlation to the histologic specimen by the dermatopathologist. These images were then stored on a secure server for use as a reference and educational tool for other diagnostic readers. We successfully achieved our goal of assisting with the development and integration of RCM and the telepathology network into the workflow of a busy private practice while building an extensive image library, thus showing potential use for other private practitioners.
Limitations of RCM
Although RCM may provide diagnostic information for many epidermal and papillary dermal lesions, it is not practical for predominantly dermal lesions or for providing prognostic information of invasive malignancies. Maximal imaging depth is 350 mm, but structures can truly be delineated at only approximately 250 μm (papillary dermis).2 Evaluation is further challenged with hypertrophic or hyperkeratotic lesions as well as those located on glabrous skin. Compared to histology, RCM resolution is slightly lower and nuclear features are not easily seen due to their weak backscattering effect.2 There are no adverse effects related to operator use; however, use may be limited if the patient has an allergy to the mediums used or to adhesive tape.
Challenges faced in integrating the technology into our practice include the machine size, time constraints, and reimbursement issues. Although not available in our office, smaller clinical devices exist (including a handheld RCM device that launched in 2007) and continue to be developed for future implementation. In our practice, capturing an image of 1 lesion took up to 20 minutes, but other protocols may necessitate only 10 minutes. Reimbursement for the imaging and image-reading procedures currently is being pursued.
Conclusion
In vivo RCM was developed as a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin. Cellular and subcellular structures as well as dynamic processes are observed without destruction of tissue. The morphologic features seen in RCM are comparable to those demonstrated with histology and dermoscopy. Despite current challenges, RCM has been shown to be an advantageous diagnostic tool, a guide to evaluating benign and malignant lesions, an adjunct to Mohs micrographic surgery via presurgical mapping of tumor margins, and a monitoring tool to establish treatment responses and efficacy. Reflectance confocal microscopy has steadily gained acceptance in clinical dermatology over the last decade, and the number of users continues to grow. With the continued efforts in advancing research, including usage of the telepathology network, we believe these tools will prove to be valuable in the private practice setting, both in the fields of dermatology and primary care.
Acknowledgment
The authors thank Caliber Imaging & Diagnostics, Inc (Rochester, New York), for providing the RCM imaging system with associated disposable supplies and the reader workstation for this review.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
1. Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
2. Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison to histology. J Invest Dermatol. 1999;113:293-303.
3. Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846-852.
4. Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
5. Astner S, González E, Cheung AC, et al. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351-359.
6. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-985.
7. González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869-874.
8. Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766-772.
9. Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862-868.
10. Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365-376.
11. Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469-474.
12. Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532-537.
13. Scope A, Benvenuto-Andrade C, Agero AL, et al. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176-185.
14. Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008:144:1597-1608.
15. Scope A, Gill M, Benveuto-Andrade C, et al. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727-734.
16. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140:1127-1132.
17. Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
18. Goldgeier M, Fox CA, Zavislan JM, et al. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol Surg. 2003;29:205-210.
19. Torres A, Niemeyer A, Berkes B, et al. 5% Imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
20. Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457-461.
21. Ruocco E, Argenziano G, Pellacani G, et al. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30(2, pt 2):301-310.
22. Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1-9.
23. Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions [published online ahead of print September 18, 2014]. J Biophotonics. doi:10.1002/jbio.201400085.
24. Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230-241.
25. Argenyl ZB. Dermoscopy (epiluminescence microscopy) of pigmented skin lesions. current status and evolving trends. Dermatologic Clin. 1997;15:79-95.
26. Grin CM, Kopf AW, Welkovich B, et al. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763-766.
27. Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
28. Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88-97.
29. Scope A, Halpern AC. Diagnostic procedures and devices. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:40-42.
30. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2008;129:131-138.
31. Junqueira LC, Carneiro J. Histology and its methods of study. In: Junqueira LC, Carneiro J, eds. Basic Histology: Text and Atlas. 11 ed. New York, NY: McGraw-Hill; 2005:1-21.
32. González S, Gill M, Halpern A, eds. Reflectance Confocal Microscopy of Cutaneous Tumors: An Atlas With Clinical, Dermoscopic and Histological Correlations. London, UK: Informa Healthcare; 2008.
33. Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
34. Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365-372.
35. Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases [published online ahead of print June 21, 2012]. J Invest Dermatol. 2012;132:2386-2394.
36. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face [published online ahead of print April 15, 2010]. J Invest Dermatol. 2010;130:2080-2091.
37. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions [published online ahead of print July 26, 2007]. J Invest Dermatol. 2007;127:2759-2765.
38. Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study [published online ahead of print October 19, 2014]. Br J Dermatol. 2014;171:1044-1051.
39. Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions [published online ahead of print July 17, 2008]. J Invest Dermatol. 2009;129:131-138
40. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
41. Malvehy J, Roldán-Marín R, Iglesias-García P, et al. Monitoring treatment of field cancerisation with 3% diclofenac sodium 2.5% hyaluronic acid by reflectance confocal microscopy: a histologic correlation. Acta Derm Venereol. 2015;95:45-50.
42. Zalaudek I, Piana S, Moscarella E, et al. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87.
43. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma tophotodynamic therapy [published online ahead of print December 13, 2012]. Dermatology. 2012;225:264-270.
Practice Points
- In vivo reflectance confocal microscopy (RCM) is a noninvasive modality for the assessment of physiologic and pathologic conditions of the skin.
- Similar to dermoscopy, RCM allows lesions to be analyzed noninvasively, and similar to histology, RCM images provide high resolution in both vertical and horizontal planes.
- Utilizing RCM as an adjunctive tool can help improve clinical diagnostic accuracy, reducing the number of biopsies of benign lesions.
- Incorporating RCM and a telepathology network into the workflow of a private practice may be valuable for dermatologists and primary care physicians.