User login
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
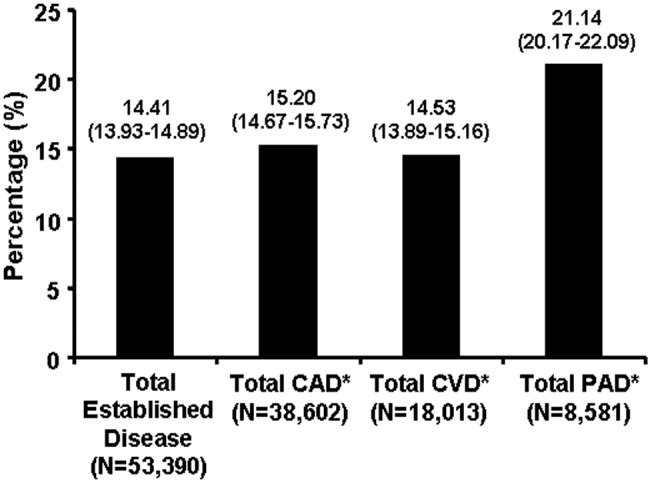
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1
Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
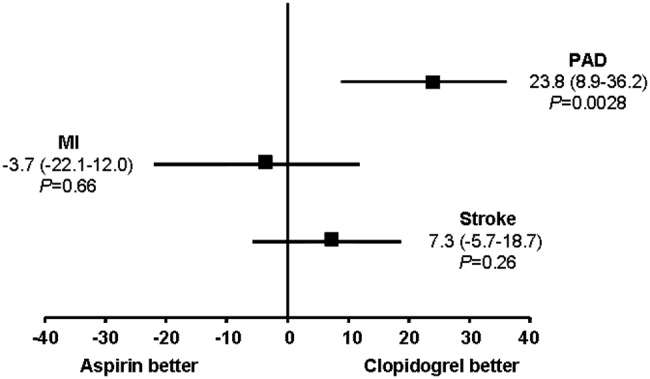
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2
CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.