User login
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
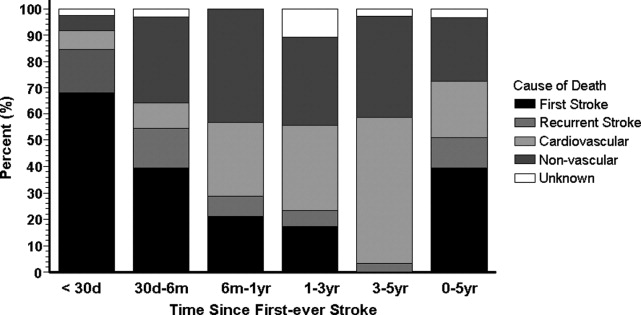
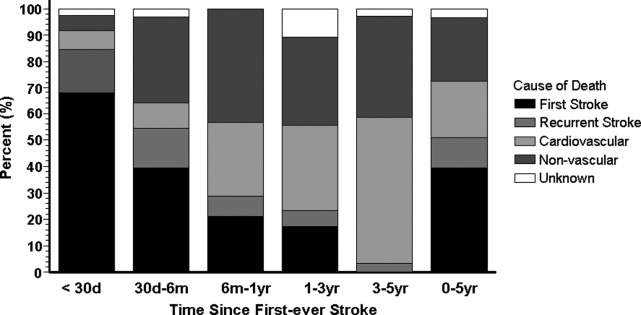
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.
MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
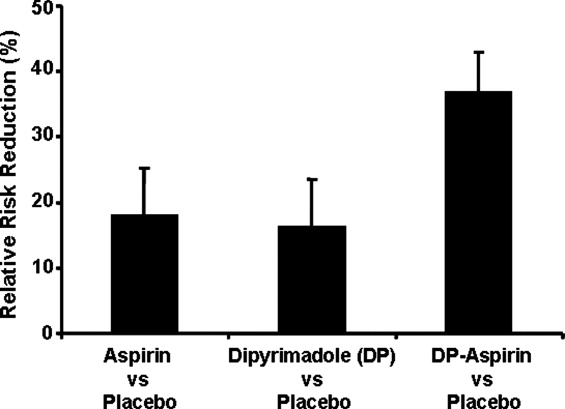
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13
The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P < .05) and a trend toward reducing all ischemic strokes (P < .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.

MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13

The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P < .05) and a trend toward reducing all ischemic strokes (P < .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.

MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13

The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P < .05) and a trend toward reducing all ischemic strokes (P < .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.