User login
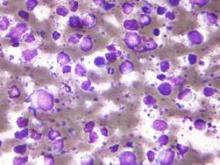
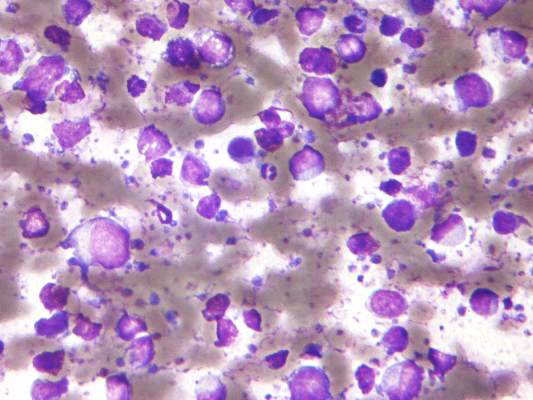
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: High doses of antimetabolites followed by R-HDS and autologous stem-cell transplantation was feasible and effective in 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
Major finding: The 2-year event-free survival rate was 50%, and the 5-year survival rate was 41%
Data source: A multicenter phase II study of 38 adults.
Disclosures: Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.