User login
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
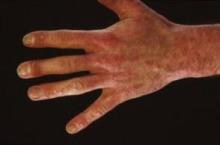
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Fifty-four percent of patients with moderate to severe hand and foot psoriasis were rated clear or had only minimal disease at those sites at week 12 after being treated with secukinumab on weeks 0, 1, 2, and 4. Only 19% of placebo-treated subjects met that standard.
Data Source: This was a post hoc secondary analysis of 131 psoriasis patients with hand and foot involvement. They were part of a larger 404-patient, double-blind, randomized phase II study.
Disclosures: The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.