User login
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
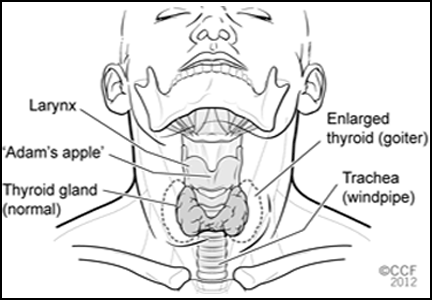
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.