User login
Subclinical hypothyroidism: When to treat
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
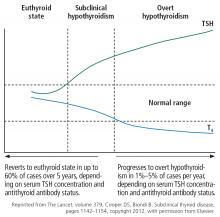
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
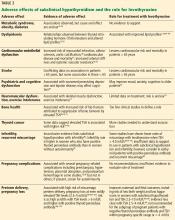
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
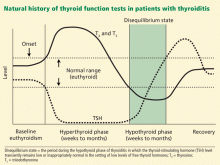
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
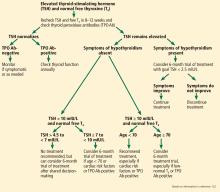
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
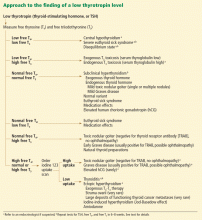
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
KEY POINTS
- From 4% to 20% of adults have subclinical hypothyroidism, with a higher prevalence in women, older people, and those with thyroid autoimmunity.
- Subclinical hypothyroidism can progress to overt hypothyroidism, especially if antithyroid antibodies are present, and has been associated with adverse metabolic, cardiovascular, reproductive, maternal-fetal, neuromuscular, and cognitive abnormalities and lower quality of life.
- Some studies have suggested that levothyroxine therapy is beneficial, but others have not, possibly owing to variability in study designs, sample sizes, and patient populations.
- Further trials are needed to clearly demonstrate the clinical impact of subclinical hypothyroidism and the effect of levothyroxine therapy.
Is a serum TSH measurement sufficient to monitor the treatment of primary hypothyroidism?
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
Insulin pumps: Great devices, but you still have to press the button
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
Should we be concerned about thyroid cancer in patients taking glucagon-like peptide 1 receptor agonists?
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
The question is complicated, as there are different types of thyroid cancer, and a causal relationship is hard to prove.
Glucagon-like peptide 1 (GLP-1) receptor agonists can be safely used in all patients with thyroid cancers that are derived from the thyroid follicular epithelium (papillary and follicular thyroid cancer). However, they are currently contraindicated in patients with medullary thyroid cancer and in patients with multiple endocrine neoplasia 2 (MEN-2), which is not a form of thyroid cancer but is relevant to our discussion. We probably should be cautious about using them in patients with familial thyroid cancer and those with a genetic predisposition for papillary or follicular thyroid cancer.
GLP-1 DRUGS ARE WIDELY USED
The glucagon-like peptide 1 (GLP-1) receptor agonists are widely used to treat type 2 diabetes mellitus. The currently available drugs of this class—exenatide (Byetta), liraglutide (Victoza), albiglutide (Tanzeum), dulaglutide (Trulicity), and extended-release exenatide (Bydureon)—are popular because they lower glucose levels, pose a low risk of hypoglycemia, can induce weight loss,1 and, in the case of extended-release exenatide and albiglutide, are given once weekly. They are currently recommended as add-on therapy to metformin. These drugs mimic the action of GLP-1, an endogenous hormone released by the intestine in response to food. They bind to receptors on beta cells, stimulating insulin production.1
FOUR TYPES OF THYROID CANCER
There are four types of thyroid cancer: medullary (a contraindication to GLP-1 agonists), papillary, follicular, and anaplastic.
Medullary thyroid cancer is extremely rare in humans, with 976 cases diagnosed from 1992 to 2006 in the United States, compared with 36,583 cases of papillary and 4,560 cases of follicular cancer. Anaplastic cancer is also rare (556 cases).2 The highest incidence rates of medullary thyroid cancer are in people of Hispanic descent (0.21 per 100,000 woman-years and 0.18 per 100,000 man-years).2
EXPERIMENTAL EVIDENCE
Pancreatic beta cells are not the only cells in the body that can express GLP-1 receptors. Notably, the parafollicular cells (also called C cells) of the thyroid, which secrete calcitonin and which are the cells involved in medullary thyroid cancer, also sometimes express these receptors if cancer develops.
In experiments in mice and rats, the incidence of thyroid C-cell tumors was higher in animals given GLP-1 analogues. Liraglutide, exenatide, taspoglutide, and lixisenatide potently activated GLP-1 receptors in thyroid C cells, increasing calcitonin gene expression and stimulating calcitonin release in a dose-dependent manner.3 Moreover, sustained activation of these receptors caused C-cell hyperplasia and resulted in medullary thyroid cancer. However medullary thyroid cancer also occurred in rodents receiving placebo.
C cells in monkeys and humans express fewer GLP-1 receptors than those in rodents; in fact, healthy human C cells do not express them at all.3,4 In rats with C-cell hyperplasia or medullary thyroid cancer, GLP-1 receptors are present in 100% of cases (and in increased density), compared with 27% of human medullary thyroid cancers.4
In addition to medullary thyroid cancer, various other human tumors have been shown to express GLP-1 receptors.5 Based on limited data, KÖrner et al5 found that these receptors are also present in various other human tumors, eg:
- Pheochromocytoma (60%)
- Paraganglioma (28%)
- Meningioma (35%)
- Astrocytoma (25%)
- Glioblastoma (9%)
- Ependymoma (16%)
- Medulloblastoma (25%)
- Nephroblastoma (22%)
- Neuroblastoma (18%)
- Ovarian adenocarcinoma (16%)
- Prostate carcinoma (5%).
Madsen et al6 reported that liraglutide binding to the GLP-1 receptor on murine thyroid C cells led to C-cell hyperplasia. However, prolonged administration of liraglutide at very high doses did not produce C-cell proliferation in monkeys.3
Gier et al7 looked at GLP-1 receptor expression in normal human C cells, hyperplastic C cells, and medullary thyroid cancer cells, as well as in papillary thyroid cancer cells, which do not arise from C cells. They demonstrated concurrent calcitonin and GLP-1 receptor immunostaining, not only in those with C-cell hyperplasia (9 of 9 cases) and medullary thyroid cancer (11 of 12 cases), but also in 3 (18%) of 17 patients with papillary thyroid cancer and 5 (33%) of 15 with normal thyroid follicular cells. However, the choice of polyclonal antibodies and radioligands used and concerns about methodology have led investigators to interpret these results cautiously.8–10
STUDIES OF GLP-1 AGONISTS IN HUMANS
Several prospective clinical studies showed no increase in calcitonin levels during therapy with GLP-1 receptor agonists in patients with type 2 diabetes.3,11 Long-term use of liraglutide in high doses (up to 3 mg per day) did not lead to elevations in serum calcitonin levels.11
In a retrospective Adverse Event Reporting System database review, the incidence rate of thyroid cancer in patients treated with exenatide was higher—with an odds ratio of 4.7 (30 events)—than with a panel of control drugs (3 events).12 However, this study did not differentiate between types of thyroid cancer, and the inherent limitations of retrospective databases complicate its interpretation. Such a high odds ratio would imply a significant increase in the incidence of medullary thyroid cancer, but this does not seem to be true.
Alves et al13 performed a meta-analysis of randomized controlled trials and long-term observational studies. None of the studies evaluating exenatide reported cases of thyroid cancer, whereas five of the studies evaluating liraglutide did. In total, nine patients treated with liraglutide were diagnosed with thyroid cancer, compared with one patient on glimepiride. The odds ratio for thyroid cancer occurrence associated with liraglutide treatment was 1.54, but that was not statistically significant (95% confidence interval 0.40–6.02, P = .53, I2 = 0%).
These studies are hypothesis-generating and do not prove that GLP-1 receptor agonists cause medullary thyroid cancer. Given the extremely low incidence of medullary thyroid cancer, to prove or disprove a causal relationship would require an enormous number of patients, who would need to be followed for several years.
OFFICIAL RECOMMENDATIONS
Considerable differences in the biology of the rodent vs human thyroid GLP-1 receptor systems have led regulatory authorities to conclude that the risk for development of medullary thyroid cancer with GLP-1 therapy in humans is difficult to quantify, but low.14 Consequently, the US Food and Drug Administration recommends neither monitoring of calcitonin levels nor ultrasound imaging as a screening tool in patients taking GLP-1 agonists.14
BENEFITS OUTWEIGH RISKS
At present, the benefits of using GLP-1 receptor agonists to treat type 2 diabetes mellitus outweigh the risks, and there seems to be little reason to withhold this effective therapy except in patients who have a personal or family history of medullary thyroid cancer or MEN-2. Until the effects of GLP-1 agonists are systematically studied in follicular-cell-derived thyroid cancer, we also recommend caution when considering their use in patients with familial thyroid cancer and those with a genetic predisposition for papillary and follicular thyroid cancer—eg, patients with familial adenomatous polyposis, phosphate and tensin homolog hamartoma tumor syndrome, Carney complex type 1, Werner syndrome, or familial papillary thyroid cancer.
Methodologically superior studies and careful long-term monitoring of patients treated with GLP-1 agonists are required to clarify the risk vs benefit of these therapies.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.
- Samson SL, Garber A. GLP-1R agonist therapy for diabetes: benefits and potential risks. Curr Opin Endocrinol Diabetes Obes 2013; 20:87–97.
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011; 21:125–134.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology 2011; 94:291–301.
- Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48:736–743.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012; 153:1538–1547.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–2031.
- Gagel RF. Activation of G-protein-coupled receptors and thyroid malignant tumors: the jury is still out. Endocr Pract 2011; 17:957–959.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141:150–156.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362:774–777.
In reply: Insulin therapy and cancer risk
In Reply: Dr. Fountas et al highlight further data on insulin therapy and cancer risk, specifically in regard to insulin detemir and insulin degludec. Detemir first gained US Food and Drug Administration (FDA) approval in 2005 as a basal insulin, dosed once or twice daily.1 Compared with regular human insulin, detemir has demonstrated proliferative and antiapoptotic activities in vitro in various cancer cell lines—eg, HCT-116 (colorectal cancer), PC-3 (prostate cancer), and MCF-7 (breast adenocarcinoma).2 But clinically, detemir has not demonstrated increased cancer risk compared with other basal insulins in randomized controlled trials or cohort studies.3–5
Degludec (U-200 insulin) is equal to twice the concentration of the usual U-100 insulin therapies presently available. In February 2013, the drug application for insulin degludec failed to obtain FDA approval, and the FDA requested additional data on cardiovascular safety. Thus, degludec is not currently available in the United States.6
Besides ameliorating nocturnal hypoglycemia,7 U-200 insulin may mitigate potential mitogenic effects.8 However, there are still very few data on degludec compared with the amount of data on insulin glargine. Insulin analogues with a decreased dissociation rate from the insulin receptor are associated with higher mitogenic potency than metabolic potency compared with human insulin.9,10 Degludec, like detemir, has an elevated dissociation rate from the insulin receptor, a low affinity for IGF-1 receptors, and a low mitogenic activity in vitro.8
At this juncture, neither detemir nor degludec has been associated with higher cancer risk, but these therapies are relatively new. And as Dr. Fountas et al indicated, their safety, particularly in regard to cancer risk in diabetes patients, should continue to be assessed.
- Levemir [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2013.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Simó R, Plana-Ripoll O, Puente D, et al. Impact of glucose-lowering agents on the risk of cancer in type 2 diabetic patients. The Barcelona case-control study. PLoS One. 2013; 8:e79968.
- Fagot JP, Blotière PO, Ricordeau P, Weill A, Alla F, Allemand H. Does insulin glargine increase the risk of cancer compared with other basal insulins? A French nationwide cohort study based on national administrative databases. Diabetes Care 2013; 36:294–301.
- Dejgaard A, Lynggaard H, Råstam J, Krogsgaard Thomsen M. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia 2009; 52:2507–2512.
- Novo Nordisk. 2013. Novo Nordisk receives Complete Response Letter in the US for Tresiba® and Ryzodeg®. [Press release]. www.novonordisk.com/include/asp/exe_news_attachment.asp?sAttachmentGUID=83700060-0ce3-4577-a35a-f3e57801637d. Accessed December 1, 2014.
- Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379:1489–1497.
- Nishimura E, Sørensen AR, Hansen BF, et al. Insulin degludec: a new ultra-long, basal insulin designed to maintain full metabolic effect while minimizing mitogenic potential. Diabetologia 2010; 53:S388–S389.
- Hansen BF, Danielsen GM, Drejer K, et al. Sustained signaling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J 1996; 315:271–279.
- Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000; 49:999–1005.
In Reply: Dr. Fountas et al highlight further data on insulin therapy and cancer risk, specifically in regard to insulin detemir and insulin degludec. Detemir first gained US Food and Drug Administration (FDA) approval in 2005 as a basal insulin, dosed once or twice daily.1 Compared with regular human insulin, detemir has demonstrated proliferative and antiapoptotic activities in vitro in various cancer cell lines—eg, HCT-116 (colorectal cancer), PC-3 (prostate cancer), and MCF-7 (breast adenocarcinoma).2 But clinically, detemir has not demonstrated increased cancer risk compared with other basal insulins in randomized controlled trials or cohort studies.3–5
Degludec (U-200 insulin) is equal to twice the concentration of the usual U-100 insulin therapies presently available. In February 2013, the drug application for insulin degludec failed to obtain FDA approval, and the FDA requested additional data on cardiovascular safety. Thus, degludec is not currently available in the United States.6
Besides ameliorating nocturnal hypoglycemia,7 U-200 insulin may mitigate potential mitogenic effects.8 However, there are still very few data on degludec compared with the amount of data on insulin glargine. Insulin analogues with a decreased dissociation rate from the insulin receptor are associated with higher mitogenic potency than metabolic potency compared with human insulin.9,10 Degludec, like detemir, has an elevated dissociation rate from the insulin receptor, a low affinity for IGF-1 receptors, and a low mitogenic activity in vitro.8
At this juncture, neither detemir nor degludec has been associated with higher cancer risk, but these therapies are relatively new. And as Dr. Fountas et al indicated, their safety, particularly in regard to cancer risk in diabetes patients, should continue to be assessed.
In Reply: Dr. Fountas et al highlight further data on insulin therapy and cancer risk, specifically in regard to insulin detemir and insulin degludec. Detemir first gained US Food and Drug Administration (FDA) approval in 2005 as a basal insulin, dosed once or twice daily.1 Compared with regular human insulin, detemir has demonstrated proliferative and antiapoptotic activities in vitro in various cancer cell lines—eg, HCT-116 (colorectal cancer), PC-3 (prostate cancer), and MCF-7 (breast adenocarcinoma).2 But clinically, detemir has not demonstrated increased cancer risk compared with other basal insulins in randomized controlled trials or cohort studies.3–5
Degludec (U-200 insulin) is equal to twice the concentration of the usual U-100 insulin therapies presently available. In February 2013, the drug application for insulin degludec failed to obtain FDA approval, and the FDA requested additional data on cardiovascular safety. Thus, degludec is not currently available in the United States.6
Besides ameliorating nocturnal hypoglycemia,7 U-200 insulin may mitigate potential mitogenic effects.8 However, there are still very few data on degludec compared with the amount of data on insulin glargine. Insulin analogues with a decreased dissociation rate from the insulin receptor are associated with higher mitogenic potency than metabolic potency compared with human insulin.9,10 Degludec, like detemir, has an elevated dissociation rate from the insulin receptor, a low affinity for IGF-1 receptors, and a low mitogenic activity in vitro.8
At this juncture, neither detemir nor degludec has been associated with higher cancer risk, but these therapies are relatively new. And as Dr. Fountas et al indicated, their safety, particularly in regard to cancer risk in diabetes patients, should continue to be assessed.
- Levemir [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2013.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Simó R, Plana-Ripoll O, Puente D, et al. Impact of glucose-lowering agents on the risk of cancer in type 2 diabetic patients. The Barcelona case-control study. PLoS One. 2013; 8:e79968.
- Fagot JP, Blotière PO, Ricordeau P, Weill A, Alla F, Allemand H. Does insulin glargine increase the risk of cancer compared with other basal insulins? A French nationwide cohort study based on national administrative databases. Diabetes Care 2013; 36:294–301.
- Dejgaard A, Lynggaard H, Råstam J, Krogsgaard Thomsen M. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia 2009; 52:2507–2512.
- Novo Nordisk. 2013. Novo Nordisk receives Complete Response Letter in the US for Tresiba® and Ryzodeg®. [Press release]. www.novonordisk.com/include/asp/exe_news_attachment.asp?sAttachmentGUID=83700060-0ce3-4577-a35a-f3e57801637d. Accessed December 1, 2014.
- Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379:1489–1497.
- Nishimura E, Sørensen AR, Hansen BF, et al. Insulin degludec: a new ultra-long, basal insulin designed to maintain full metabolic effect while minimizing mitogenic potential. Diabetologia 2010; 53:S388–S389.
- Hansen BF, Danielsen GM, Drejer K, et al. Sustained signaling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J 1996; 315:271–279.
- Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000; 49:999–1005.
- Levemir [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2013.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Simó R, Plana-Ripoll O, Puente D, et al. Impact of glucose-lowering agents on the risk of cancer in type 2 diabetic patients. The Barcelona case-control study. PLoS One. 2013; 8:e79968.
- Fagot JP, Blotière PO, Ricordeau P, Weill A, Alla F, Allemand H. Does insulin glargine increase the risk of cancer compared with other basal insulins? A French nationwide cohort study based on national administrative databases. Diabetes Care 2013; 36:294–301.
- Dejgaard A, Lynggaard H, Råstam J, Krogsgaard Thomsen M. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia 2009; 52:2507–2512.
- Novo Nordisk. 2013. Novo Nordisk receives Complete Response Letter in the US for Tresiba® and Ryzodeg®. [Press release]. www.novonordisk.com/include/asp/exe_news_attachment.asp?sAttachmentGUID=83700060-0ce3-4577-a35a-f3e57801637d. Accessed December 1, 2014.
- Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379:1489–1497.
- Nishimura E, Sørensen AR, Hansen BF, et al. Insulin degludec: a new ultra-long, basal insulin designed to maintain full metabolic effect while minimizing mitogenic potential. Diabetologia 2010; 53:S388–S389.
- Hansen BF, Danielsen GM, Drejer K, et al. Sustained signaling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J 1996; 315:271–279.
- Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000; 49:999–1005.
In reply: Diabetes therapy and cancer risk
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
Diabetes therapy and cancer risk: Where do we stand when treating patients?
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
In the last quarter century, many new drugs have become available for treating type 2 diabetes mellitus. The American Association of Clinical Endocrinologists incorporated these new agents in its updated glycemic control algorithm in 2013.1 Because diabetes affects 25.8 million Americans and can lead to blindness, renal failure, cardiovascular disease, and amputation, agents that help us treat it more effectively are valuable.2
One of the barriers to effective treatment is the side effects of the agents. Because some of these drugs have been in use for only a short time, concerns of potential adverse effects have arisen. Cancer is one such concern, especially since type 2 diabetes mellitus by itself increases the risk of cancer by 20% to 50% compared with no diabetes.3
Type 2 diabetes has been linked to risk of cancers of the pancreas,4 colorectum,5,6 liver,7 kidney,8,9 breast,10 bladder,11 and endometri-um,12 as well as to hematologic malignancies such as non-Hodgkin lymphoma.13 The risk of bladder cancer appears to depend on how long the patient has had type 2 diabetes. Newton et al,14 in a prospective cohort study, found that those who had diabetes for more than 15 years and used insulin had the highest risk of bladder cancer. On the other hand, the risk of prostate cancer is actually lower in people with diabetes,15 particularly in those who have had diabetes for longer than 4 years.16
Cancer and type 2 diabetes share many risk factors and underlying pathophysiologic mechanisms. Nonmodifiable risk factors for both diseases include advanced age, male sex, ethnicity (African American men appear to be most vulnerable to both cancer and diabetes),17,18 and family history. Modifiable risk factors include lower socioeconomic status, obesity, and alcohol consumption. These common risk factors lead to hyperinsulinemia and insulin resistance, changes in mitochondrial function, low-grade inflammation, and oxidative stress,3 which promote both diabetes and cancer. Diabetes therapy may influence several of these processes.
Several classes of diabetes drugs, including exogenous insulin,19–22 insulin secretagogues,23–25 and incretin-based therapies,26–28 have been under scrutiny because of their potential influences on cancer development in a population already at risk (Table 1).
INSULIN ANALOGUES: MIXED EVIDENCE
Insulin promotes cell division by binding to insulin receptor isoform A and insulin-like growth factor 1 receptors.29 Because endogenous hyperinsulinemia has been linked to cancer risk, growth, and proliferation, some speculate that exogenous insulin may also increase cancer risk.
In 2009, a retrospective study by Hemkens et al linked the long-acting insulin analogue glargine to risk of cancer.19 This finding set off a tumult of controversy within the medical community and concern among patients. Several limitations of the study were brought to light, including a short duration of follow-up, and several other studies have refuted the study’s findings.20,21
More recently, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial22 found no higher cancer risk with glargine use than with placebo. This study enrolled 12,537 participants from 573 sites in 40 countries. Specifically, risks with glargine use were as follows:
- Any cancer—hazard ratio 1.00, 95% confidence interval (CI) 0.88–1.13, P = .97
- Cancer death—hazard ratio 0.94, 95% CI 0.77–1.15, P = .52.
However, the study was designed to assess cardiovascular outcomes, not cancer risk. Furthermore, the participants were not typical of patients seen in clinical practice: their insulin doses were lower (the median insulin dose was 0.4 units/kg/day by year 6, whereas in clinical practice, those with type 2 diabetes mellitus often use more than 1 unit/kg/day, depending on duration of diabetes, diet, and exercise regimen), and their baseline median hemoglobin A1c level was only 6.4%. And one may argue that the median follow-up of 6.2 years was too short for cancer to develop.22
In vitro studies indicate that long-acting analogue insulin therapy may promote cancer cell growth more than endogenous insulin,30 but epidemiologic data have not unequivocally substantiated this.20–22 There is no clear evidence that analogue insulin therapy raises cancer risk above that of human recombinant insulin, and starting insulin therapy should not be delayed because of concerns about cancer risk, particularly in uncontrolled diabetes.
INSULIN SECRETAGOGUES
Sulfonylureas: Higher risk
Before 1995, only two classes of diabetes drugs were approved by the US Food and Drug Administration (FDA)—insulin and sulfonylureas.
Sulfonylureas lower blood sugar levels by binding to sulfonylurea receptors and inhibiting adenosine triphosphate-dependent potassium channels. The resulting change in resting potential causes an influx of calcium, ultimately leading to insulin secretion.
Sulfonylureas are effective, and because of their low cost, physicians often pick them as a second-line agent after metformin.
The main disadvantage of sulfonylureas is the risk of hypoglycemia, particularly in patients with renal failure, the elderly, and diabetic patients who are unaware of when they are hypoglycemic. Other potential drawbacks are that they impair cardiac ischemic preconditioning31 and possibly increase cancer risk.21,32 (Ischemic preconditioning is the process in which transient episodes of ischemia “condition” the myocardium so that it better withstands future episodes with minimal anginal pain and tissue injury.33) Of the sulfonylureas, glyburide has been most implicated in cardiovascular risk.32
In a retrospective cohort study of 62,809 patients from a general-practice database in the United Kingdom, Currie et al21 found that sulfonylurea monotherapy was associated with a 36% higher risk of cancer (95% CI 1.19–1.54, P < .001) than metformin monotherapy. Prescribing bias may have influenced the results: practitioners are more likely to prescribe sulfonylureas to leaner patients, who have a greater likelihood of occult cancer. However, other studies also found that the cancer death rate is higher in those who take a sulfonylurea alone than in those who use metformin alone.23,24
Some evidence indicates that long-acting sulfonylurea formulations (eg, glyburide) likely hold the most danger, certainly in regard to hypoglycemia, but it is less clear if this translates to cancer concerns.31
Meglitinides: Limited evidence
Meglitinides, the other class of insulin secretagogues, are less commonly used but are similar to sulfonylureas in the way they increase endogenous insulin levels. The data are limited regarding cancer risk and meglitinide therapy, but the magnitude of the association is similar to that with sulfonylurea therapy.25
INSULIN SENSITIZERS
There are currently two classes of insulin sensitizers: biguanides and thiazolidinediones (TZDs, also known as glitazones). These drugs show less risk of both cancer incidence and cancer death than insulin secretagogues such as sulfonylureas.21,23,24 In fact, they may decrease cancer potential by alteration of signaling via the AKT/mTOR (v-akt murine thymoma viral oncogene homolog 1/mammalian target of rapamycin) pathway.34
Metformin, a biguanide, is the oral drug of choice
Metformin is the only biguanide currently available in the United States. It was approved by the FDA in 1995, although it had been in clinical use since the 1950s. Inexpensive and familiar, it is the oral antihyperglycemic of choice if there are no contraindications to it, such as renal dysfunction (creatinine ≥ 1.4 mg/dL in women and ≥ 1.5 mg/dL in men), acute decompensated heart failure, or pulmonary or hepatic insufficiency, all of which may lead to an increased risk of lactic acidosis.1
Metformin lowers blood sugar levels primarily by inhibiting hepatic glucose production (gluconeogenesis) and by improving peripheral insulin sensitivity. It directly activates AMP-activated protein kinase (AMPK), which affects insulin signaling and glucose and fat metabolism.35 It may exert further beneficial effects by acutely increasing glucagon-like peptide-1 (GLP-1) levels and inducing islet incretin-receptor gene expression.36 Although the exact mechanisms have not been fully elucidated, metformin’s insulin-sensitizing properties are likely from favorable effects on insulin receptor expression, tyrosine kinase activity, and influences on the incretin pathway.36,37 These effects also mitigate carcinogenesis, both directly (via AMPK and liver kinase B1, a tumor-suppressor gene) and indirectly (via reduction of hyperinsulinemia).35
Overall, biguanide therapy is associated with a lower cancer incidence or, at worst, no effect on cancer incidence. In vitro studies demonstrate that metformin both suppresses cancer cell growth and induces apoptosis, resulting in fewer live cancer cells.34 Several retrospective studies found lower cancer risk in metformin users than in patients receiving antidiabetes drugs other than insulin-sensitizing agents,21,23,25,38–40 while others have shown no effect.41 Use of metformin was specifically associated with lower risk of cancers of the liver, colon and rectum, and lung.42 Further, metformin users have a lower cancer mortality rate than nonusers.24,43
Thiazolidinediones
TZDs, such as pioglitazone, work by binding to peroxisome proliferator-activated gamma receptors in the cell nucleus, altering gene transcription.44 They reduce insulin resistance and levels of endogenous insulin levels and free fatty acids.44
Concern over bladder cancer risk with TZD use, particularly with pioglitazone, has increased in the last few years, as various cohort studies found a statistically significant increased risk with this agent.44 The risk appears to rise with cumulative dose.45,46
Randomized controlled trials also found an increased risk of bladder cancer with TZD therapy, although the difference was not statistically significant.47–49 In a mean follow-up of 8.7 years, the Prospective Pioglitazone Clinical Trial in Macrovascular Events reported 23 cases of bladder cancer in the pioglitazone group vs 22 cases in the placebo group, for rates of 0.9% vs 0.8% (relative risk [RR] 1.06, 95% CI 0.59–1.89).49
On the other hand, the risk of cancer of the breast, colon, and lung has been found to be lower with TZD use.47 In vitro studies support the clinical data, showing that TZDs inhibit growth of human cancer cells derived from cancers of the lung, colon, breast, stomach, ovary, and prostate.50–53
Home et al54 compared rosiglitazone against a sulfonylurea in patients already taking metformin in the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial. Malignancies developed in 6.7% of the sulfonylurea group compared with 5.1% of the rosiglitazone group, for a hazard ratio of 1.33 (95% CI 0.94–1.88).
Both ADOPT (A Diabetes Outcome Progression Trial) and the RECORD trial found rosiglitazone comparable to metformin in terms of cancer risk.54
Colmers et al47 pooled data from four randomized controlled trials, seven cohort studies, and nine case-control studies to assess the risk of cancer with TZD use in type 2 diabetes. Both the randomized and observational data showed neutral overall cancer risk with TZDs. However, pooled data from observational studies showed significantly lower risk with TZD use in terms of:
- Colorectal cancer RR 0.93, 95% CI 0.87–1.00
- Lung cancer RR 0.91, 95% CI 0.84–0.98
- Breast cancer RR 0.89, 95% CI 0.81–0.98.
INCRETIN-BASED THERAPIES
Incretins are hormones released from the gut in response to food ingestion, triggering release of insulin before blood glucose levels rise. Their action explains why insulin secretion increases more after an oral glucose load than after an intravenous glucose load, a phenomenon called the incretin effect.55
There are two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). They have short a half-life because they are rapidly degraded by dipeptidyl peptidase-IV (DPP-IV).55 Available incretin-based therapies are GLP-1 receptor agonists and DPP-IV inhibitors.
When used as monotherapy, incretin-based therapies do not cause hypoglycemia because their effect is glucose-dependent.55 GLP-1 receptor antagonists have the added benefit of inducing weight loss, but DPP-IV inhibitors are considered to be weight-neutral.
GLP-1 receptor agonists
Exenatide, the first of the GLP-1 receptor agonists, was approved in 2005. The original formulation (Byetta) is taken by injection twice daily, and timing in conjunction with food intake is important: it should be taken within 60 minutes before the morning and evening meals. Extended-release exenatide (Bydureon) is a once-weekly formulation taken without regard to timing of food intake. Exenatide (either twice-daily Byetta or once-weekly Bydureon) should not be used in those with creatinine clearance less than 30 mL/min or end-stage renal disease and should be used with caution in patients with renal transplantation.
Liraglutide (Victoza), a once-daily formulation, can be injected irrespective of food intake. The dose does not have to be adjusted for renal function, although it should be used with caution in those with renal impairment, including end-stage renal disease. Approval for a 3-mg formulation is pending with the FDA as a weight-loss drug on the basis of promising results in a randomized phase 3 trial.56
Albiglutide (Tanzeum), a once-weekly GLP-1 receptor antagonist, was recently approved by the FDA.
DPP-IV inhibitors
Whereas GLP-1 receptor agonists are injected, the DPP-IV inhibitors have the advantage of being oral agents.
Sitagliptin (Januvia), the first DPP-IV inhibitor, became available in the United States in 2006. Since then, three more have become available: saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).
Concerns about thyroid cancer with incretin drugs
Concerns of increased risk of cancer, particularly of the thyroid and pancreas, have been raised since GLP-1 receptor agonists and DPP-IV inhibitors became available.
Studies in rodents have shown C-cell hyperplasia, sometimes resulting in increased incidence of thyroid carcinoma, and dose-dependent rises in serum calcitonin, particularly with liraglutide.26 This has raised concern about an increased risk of medullary thyroid carcinoma in humans. However, the density of C cells in rodents is up to 45 times greater than in humans, and C cells also express functional GLP-1 receptors.26
Gier et al27 assessed the expression of calcitonin and human GLP-1 receptors in normal C cells, C cell hyperplasia, and medullary cancer. In this study, calcitonin and GLP-1 receptor were co-expressed in medullary thyroid cancer (10 of 12 cases) and C-cell hyperplasia (9 of 9 cases) more commonly than in normal C cells (5 of 15 cases). Further, GLP-1 receptor was expressed in 3 of 17 cases of papillary thyroid cancer.
Calcitonin, a polypeptide hormone produced by thyroid C cells and used as a medullary thyroid cancer biomarker, was increased in a slightly higher percentage of patients treated with liraglutide than in controls, without an increase above the normal range.57
A meta-analysis by Alves et al58 of 25 studies found that neither exenatide (no cases reported) nor liraglutide (odds ratio 1.54, 95% CI 0.40–6.02) was associated with increased thyroid cancer risk.
MacConell et al59 pooled the results of 19 placebo-controlled trials of twice-daily exenatide and found a thyroid cancer incidence rate of 0.3 per 100 patient-years (< 0.1%) vs 0 per 100 patient-years in pooled comparators.
Concerns about pancreatic cancer with incretin drugs
Increased risk of acute pancreatitis is a potential side effect of both DPP-IV inhibitors and GLP-1 receptor agonists and has led to speculation that this translates to an increased risk of pancreatic cancer.
In a point-counterpoint debate, Butler et al28 argued that incretin-based medications have questionable safety, with increased rates of pancreatitis possibly leading to pancreatic cancer. In counterpoint, Nauck60 argued that the risk of pancreatitis or cancer is extremely low, and clinical cases are unsubstantiated.
Bailey61 outlined the complexities and difficulties in drawing firm conclusions from individual clinical trials regarding possible adverse effects of diabetes drugs. The trials are typically designed to assess hemoglobin A1c reduction at varying doses and are typically restricted in patient selection, patient numbers, and drug-exposure duration, which may introduce allocation and ascertainment biases. The attempt to draw firm conclusions from such trials can be problematic and can lead to increased alarm, warranted or not.
Type 2 diabetes mellitus itself is associated with an increased incidence of pancreatic cancer, and whether incretin therapy enhances this risk is still controversial. Whether more episodes of acute pancreatitis without chronic pancreatitis can be extrapolated to an increased incidence of pancreatic cancer is doubtful. A normal pancreatic duct cell may take up to 12 years to become a tumor cell from which pancreatic carcinoma develops, another 7 years to develop metastatic capacity, and another 3 years before a diagnosis is made from clinical symptoms (which are usually accompanied by metastases).62
The risks and benefits of incretin therapies remain a contentious issue, and there are no clear prospective data at this time on increased pancreatic cancer incidence. Long-term prospective studies designed to analyze these specific outcomes (pancreatitis, pancreatic cancer, and medullary thyroid cancer) need to be undertaken.63
OTHER DIABETES THERAPIES
Alpha glucosidase inhibitors
Oral glucosidase inhibitors ameliorate hyperglycemia by inhibiting alpha glucosidase enzymes in the brush border of the small intestines, preventing conversion of polysaccharides to monosaccharides.64 This slows digestion of carbohydrates and glucose release into the bloodstream and blunts the postprandial hyperglycemic excursion.
The two alpha glucosidase inhibitors currently available in the United States are acarbose and miglitol, and although data are limited, they do not appear to increase the risk of cancer.65,66
Sodium-glucose-linked cotransporter 2 inhibitors
The newest class of oral diabetes agents to be approved are the sodium-glucose-linked cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga).
SGLT2 is a protein in the S1 segment of the proximal renal tubules responsible for over 90% of renal glucose reabsorption. SGLT2 inhibitors lower serum glucose levels by promoting glycosuria and have also been shown to have favorable effects on blood pressure and weight.67,68
Canagliflozin was the first of its class to gain FDA approval in the United States. It has not been found to be associated with increased cancer risk.68
Dapagliflozin, originally approved in Europe, was approved in the United States on January 8, 2014. Because of a possible increased incidence of breast and bladder malignancies, the FDA advisory committee initially recommended against approval and required further data. In those who were treated, nine cases of bladder cancer and nine cases of breast cancer were reported, compared with one case of bladder cancer and no cases of breast cancer in the control group; however, the difference was not statistically significant.68
Since SGLT2 inhibitors are still new, data on long-term outcomes are lacking. Early clinical data do not show a significant increase in cancer risk.
WHAT THIS MEANS IN PRACTICE
Many studies have found associations between diabetes, obesity, hyperinsulinemia, and cancer risk. In the last decade, concerns implicating antihyperglycemic agents in cancer development have arisen but have not been well substantiated. At this time, there are no definitive prospective data indicating that the currently available type 2 diabetes therapies increase the incidence of cancer beyond the inherent increased risk in this population. What, then, is one to do?
Educate. Lifestyle modification, including weight management, should continue to be emphasized in diabetes education, as no therapy is completely effective without adjunct modifications in diet and physical activity. Epidemiologic studies have shown the benefits of lifestyle modifications, which ameliorate many of the adverse metabolic conditions that coexist in type 2 diabetes and cancer.
Screen for cancer. Given the associations between diabetes and malignancy, cancer screening is especially important in this high-risk population.
Customize therapy to individual patients. Those with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid sitagliptin until definitive clinical data become available.
Moreover, patients with a personal or family history of medullary thyroid cancer should not receive GLP-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma. Until we have further elucidating data, it is not possible to say whether a family history of any of the other types of cancer should represent a contraindication to the use of any of these agents.
Discuss. The multitude of diabetes therapies warrants physician-patient discussions that carefully weigh the risks and benefits of additional agents to optimize glycemic control and metabolic factors in individual patients.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Centers for Disease Control and Prevention (CDC). Diabetes data and trends. www.cdc.gov/diabetes/statistics/. Accessed April 8, 2014.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687.
- Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006; 101:1872–1879.
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380.
- Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112.
- Washio M, Mori M, Khan M, et al; JACC Study Group. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 2007; 14:393–397.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823.
- Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007; 50:1365–1374.
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 2008; 31:2391–2397.
- Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 2013; 132:2186–2191.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:2056–2062.
- Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005; 161:147–152.
- Centers for Disease Control and Prevention. Diabetes public health resource. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pubs/estimates14.htm. Accessed August 12, 2014.
- Centers for Disease Control and Prevention. Cancer prevention and control cancer rates by race and ethnicity. www.cdc.gov/cancer/dcpc/data/race.htm. Accessed August 12, 2014.
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; 52:1732–1744.
- Colhoun HMSDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; 52:1755–1765.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Baur DM, Klotsche J, Hamnvik OP, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011; 60:1363–1371.
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29:254–258.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137:482–488.
- Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013; 36:2118–2125.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011; 18:R125–R147.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009; 25:41–49.
- Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab 2003; 88:528–530.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012; 107:620–626.
- Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrmann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990; 82:2044–2051.
- Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med 2011; 15:825–836.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122:253–270.
- Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011; 54:339–349.
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323–1332.
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011; 123:200–204.
- Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011; 20:337–344.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7:e33411.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
- Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344:e3645.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2012; 38:475–484.
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32:187–202.
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A, Perez A. Pioglitazone and bladder malignancy during observational follow-up of PROactive: 6-year update. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA.
- Akinyeke TO, Stewart LV. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol Ther 2011; 11:1046–1058.
- Ban JO, Oh JH, Son SM, et al. Troglitazone, a PPAR agonist, inhibits human prostate cancer cell growth through inactivation of NFKB via suppression of GSK-3B expression. Cancer Biol Ther 2011; 12:288–296.
- Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog 2010; 49:235–246.
- Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2010; 10:390.
- Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti GADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845.
- Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 41:299–307.
- Wadden TA, Hollander P, Klein S, et al; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37:1443–1451.
- Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96:853–860.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98:271–284.
- MacConell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5,594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5:29–41.
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013; 36:2126–2132.
- Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care 2013; 36:2098–2106.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467:1114–1117.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 1995; 18:303–311.
- Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009; 46:279–284.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011; 54:2009–2015.
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022.
- Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5:313–527.
KEY POINTS
- Exogenous insulin, insulin secretagogues, and incretin-based therapies are under scrutiny because of their potential influences on cancer development in a population already at risk.
- At present, we lack adequate prospective data on the cancer risk from diabetes drugs.
- Patients with a personal history of bladder cancer should avoid pioglitazone, and those who have had pancreatic cancer should avoid incretin therapies until definitive clinical data become available.
- Patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 should not receive glucagon-like peptide-1 receptor agonists. These agents should also probably be avoided in patients with a personal history of differentiated thyroid carcinoma or a history of familial nonmedullary thyroid carcinoma.
- Given the associations between diabetes and malignancy, cancer screening is especially important.
Approach to a low TSH level: Patience is a virtue
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
KEY POINTS
- A low TSH value should always be followed up by measuring the thyroid hormones, ie, thyroxine (T4) and triiodothyronine (T3).
- Serum levels of free thyroid hormones should be used when interpreting an abnormal TSH level, especially in the acute and inpatient settings.
- A low TSH level is not always the result of suppression by elevations in circulating thyroid hormones.
- A low TSH level in the setting of normal levels of free thyroid hormones should always be reassessed in 4 to 6 weeks before making a diagnosis.
- Overt hyperthyroidism is usually associated with a frankly suppressed TSH (< 0.1 μIU/mL).