User login
In reply: Diabetes therapy and cardiac risk
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
Approach to a low TSH level: Patience is a virtue
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
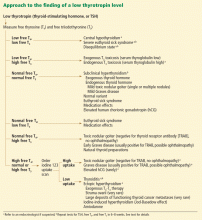
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
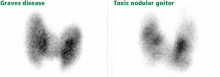
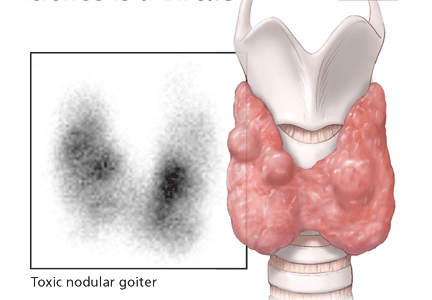
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
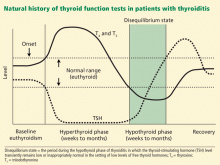
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
A 34-year-old woman presents to the outpatient endocrinology clinic 4 months postpartum. She says that 2 months ago she developed palpitations, heat intolerance, and difficulty sleeping. Her primary care physician diagnosed postpartum thyroiditis after laboratory evaluation revealed that her thyrotropin (thyroid-stimulating hormone, TSH) level was low at 0.005 μIU/mL (reference range 0.4–5.5), and that her free thyroxine (T4) level was elevated at 2.4 ng/dL (reference range 0.7–1.8). She was prescribed atenolol (Tenormin) to treat the symptoms.
On follow-up testing 6 weeks later, her TSH level had risen, but it was still low at 0.085 μIU/mL, and her free T4 level was now low at 0.6 ng/dL. She was referred to an endocrinologist for further management.
How should this patient be further evaluated and managed?
LOW TSH HAS MANY CAUSES
Follow up the finding of a low TSH by measuring free T4 and free T3
The finding of a low TSH level should always be followed up by measuring the thyroid hormones, ie, T4 and triiodothyronine (T3).
The levels of free T4 and free T3 should be used, not total levels, when interpreting an abnormal TSH value. This especially applies in the acute and inpatient settings, in which many patients are malnourished and consequently have low serum levels of thyroid-binding globulin and albumin. In this situation, total T4 and T3 levels may be low and not accurately represent a patient’s true thyroid status. Likewise, in women who are pregnant or taking an estrogen-containing contraceptive, the total T4 and T3 levels may be high, secondary to an increase in thyroid-binding globulin synthesis, but the free T4 and free T3 are normal (in the absence of a pathologic process).
However, depending on the analytical method, even measurements of the free hormones may be affected by the protein changes that occur during severe illness or pregnancy. Also, some drugs can affect free hormone levels by displacing the hormones from their binding proteins.
Most commercial laboratories estimate the levels of free thyroid hormones by indirect methods. Short of measuring the free thyroid hormones directly using equilibrium dialysis and ultrafiltration (the gold standard), no test or assay is 100% accurate. Even the determination of free hormone levels can be flawed if the assay is unreliable. Some clinicians still prefer the free thyroid index (FTI) and T3 or T4 resin uptake to assess free T4, and the total T3 to assess T3 status.
The degree of TSH suppression should also be taken into account. A frankly suppressed TSH level (< 0.1 μIU/mL) would favor overt thyrotoxicosis in the correct clinical context (ie, if the levels of free T4, free T3, or both were normal or high).
Figure 1 outlines how to interpret a low TSH level and formulate the appropriate diagnosis and plan. In this process, it is crucial to consider the patient’s history, to note signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism), and to ask about medication exposure. Furthermore, repeating the thyroid function tests (and reviewing previous values) to observe the trend is consistently invaluable when deriving a diagnosis.
LOW TSH, LOW FREE T4, LOW FREE T3
The history of present illness (especially if the illness is prolonged and critical), a review of previous thyroid function tests, and, sometimes, a complete evaluation of the remaining hypothalamic-pituitary axes are crucial in correctly interpreting this combination of thyroid function tests. Clinical judgment is required, and referral to an endocrinologist is warranted. The diagnostic possibilities are:
Central hypothyroidism. A low TSH level is not always due to suppression caused by high thyroid hormone levels, other conditions, or medications. If thyroid hormone levels are low, a low TSH value can be the result of a central process (hypothalamic or pituitary or both).
Severe euthyroid sick syndrome (also called “nonthyroidal illness” or “low T3 syndrome”). In this condition, the free T3 level is usually low, and in severe cases the free T4 level can also be low.1,2
LOW TSH, LOW FREE T4, HIGH FREE T3
T3 toxicosis from an exogenous source
The combination of low TSH, low free T4, and elevated free T3 concentrations is consistent with ingestion of supratherapeutic doses of exogenous T3, ie, liothyronine (Cytomel).
Rarely is T3 therapy used alone to treat hypothyroidism. An exception is in patients who undergo thyroid hormone withdrawal in anticipation of radioactive iodine treatment after having undergone total thyroidectomy for differentiated thyroid cancer.
T3 therapy, when used, is often given in combination with T4 therapy, either levothyroxine (Synthroid and others) or as part of a T4-T3 natural thyroid preparation derived from porcine thyroid tissue (Armour Thyroid, Nature-Throid). Natural thyroid preparations may contain large amounts of T3, and when they are given in supratherapeutic doses, they can cause a similar profile (low TSH, low free T4, and elevated free T3). However, the free T4 level is usually in the normal range because the preparations also contain T4.
T3 toxicosis from an endogenous source
Sometimes the thyroid gland produces disproportionately large amounts of T3, usually from an autonomous nodule. Although the free T4 level may be low in this situation, it is usually in the normal range.
Serum thyroglobulin can be assayed to help determine whether the source of excess T3 is exogenous (in which case the thyroglobulin level is low) or endogenous (in which case the thyroglobulin is elevated). If it is endogenous, the patient should be referred to an endocrinologist for further evaluation.
LOW TSH, NORMAL FREE T4, NORMAL FREE T3
Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined as low TSH, normal free T4, and normal free T3 levels. Symptoms of hyperthyroidism such as fatigue, insomnia, weight loss, palpitations, tremor, or heat intolerance generally play a role in whether therapy is considered, but not in making the diagnosis of subclinical hyperthyroidism. To make the correct diagnosis, it is crucial to confirm that this pattern of test results persists by repeating these tests over the next few months.
Exogenous thyrotoxicosis, by far the most common form of subclinical thyrotoxicosis, results from taking levothyroxine (T4) or liothyronine (T3), or both, either in unintentional supratherapeutic doses in patients with hypothyroidism or in intentionally high doses to suppress TSH in patients with a history of differentiated thyroid cancer.
Endogenous thyrotoxicosis. Subclinical hyperthyroidism from an endogenous cause is the result of an underlying pathophysiologic process, the same processes responsible for overt states of hyperthyroidism (eg, Graves disease, toxic nodular thyroid disease) (see the discussion of overt hyperthyroidism in a later section).
The course of endogenous subclinical hyperthyroidism depends on the underlying cause and on the level of TSH suppression.3–5 Subclinical hyperthyroidism secondary to a multinodular goiter is estimated to progress to overt hyperthyroidism in about 5% of patients per year,6 but in patients with nodular thyroid disease and TSH levels of 0.1 μIU/mL or lower, one study reported progression to overt hyperthyroidism in approximately 10% of patients per year.3
The risk of subclinical Graves disease progressing to overt hyperthyroidism has been difficult to estimate, given the relapsing and remitting nature of the disease. Rosario3,4 reported that subclinical Graves disease progressed to overt hyperthyroidism in 2 years in 6 (40%) of 15 patients who had TSH levels lower than 0.1 μIU/mL, but in no patients who had TSH levels of 0.1 to 0.4 μIU/mL. These patients were younger than 65 years. In a group age 60 and older with endogenous subclinical hyperthyroidism and a TSH level between 0.1 and 0.4 μIU/mL, Rosario4 reported that progression to overt hyperthyroidism was uncommon, occurring in about 1% of patients per year.
Thus, periodic reassessment of thyroid function tests in patients with subclinical hyperthyroidism is crucial in monitoring for disease progression, especially in those with frankly suppressed TSH values (< 0.1 μIU/mL).
Adverse outcomes associated with subclinical hyperthyroidism are mainly cardiac arrhythmias (atrial fibrillation) and accelerated loss of bone mineral density.
Cooper7 notes that definitive treatment (radioactive iodine ablation, antithyroid drugs, or surgery) “seems reasonable” for older patients (age > 60 years) with a TSH level lower than 0.1 μIU/mL and for certain patients with TSH levels of 0.1 to 0.4 who are at high risk, eg, those with a history of heart disease, osteoporosis, or symptoms of hyperthyroidism.
Normal variant
The normal range for TSH, as for other substances, is defined as the mean value in the general population plus or minus 2 standard deviations. This range includes 95% of the population, so that 2.5% of people have a level higher than this range, and 2.5% have a level lower than this range.
But some people with lower levels of TSH, especially in the range of 0.1 to 0.4 μIU/mL (3 standard deviations below the mean) are actually euthyroid. These people have historically been classified as having subclinical hyperthyroidism, as there is no means of differentiating these “normal” euthyroid people from people with asymptomatic subclinical hyperthyroidism. They need to be followed, since they may have true subclinical hyperthyroidism that may manifest symptomatically in the future, possibly warranting treatment.
Euthyroid sick syndrome
Euthyroid sick syndrome is common during critical illness. However, thyroid disease is common in the general population, and often no test results from before the onset of a critical illness are available to help the clinician separate overt thyroid disease from euthyroid sick syndrome. Furthermore, patients are often unable to provide a history (or to relate their symptoms) of overt thyroid disease, making abnormal thyroid function tests difficult to interpret in the hospital. When previous values are available, they can be invaluable in correctly interpreting new abnormal results.
Thyroid function test values in euthyroid sick syndrome can vary depending on the severity of illness. A low free T3, a normal free T4, and a low-normal TSH are the most common abnormalities seen in euthyroid sick syndrome. The free T3 level is low because of decreased peripheral conversion of T4 to T3 during critical illness. However, euthyroid sick syndrome can present with a spectrum of abnormal thyroid function tests, further complicating interpretation and diagnosis. Serum TSH levels have been reported to be normal in about 50%, low in 30%, and high in 12% of patients with nonthyroidal illness.8 However, marked suppression of serum TSH (< 0.1 μIU/mL) was observed only in about 7% of patients, mainly in those whose clinical picture was confounded by medications (dopamine or corticosteroids, or both) that have independent TSH-lowering effects (see below).8
Drugs that suppress TSH
Many drugs used in the hospital and intensive care unit can alter thyroid function tests independently of systemic illness, further complicating the clinical picture.
Glucocorticoids, in high doses, have been shown to transiently suppress serum TSH.9,10
Octreotide (Sandostatin) and other somatostatin analogues also transiently suppress TSH.11–14 However, these drugs (and glucocorticoids) do not appear to result in central hypothyroidism.10,15–17
Dopamine, given in pharmacologic doses for a prolonged time, has been shown to reduce the serum TSH level in both critically ill and normal healthy people.18
Dobutamine (Dobutrex) in pharmacologic doses has been likewise shown to lower TSH levels, although the serum TSH level was noted to remain within the normal range in those who had a normal TSH value at baseline.19
Amiodarone. Although most patients who take amiodarone (Cordarone, Pacerone) remain euthyroid, the drug can cause hypothyroidism or hyperthyroidism. Initially, amiodarone usually causes a decrease in T3 via inhibition of 5′-deiodinase, with a transient reciprocal increase in TSH.20
When amiodarone induces thyrotoxicosis, the condition can be subclinical, manifested by a low TSH in the setting of normal levels of thyroid hormones, or as overt thyrotoxicosis with a low TSH and elevated levels of thyroid hormones. See further discussion below on amiodarone’s effects on thyroid function.
Patients taking drugs that lower TSH are often critically ill and may also have a component of euthyroid sick syndrome, resulting in a mixed picture.
Elevated human chorionic gonadotropin
The alpha subunit of human chorionic gonadotropin (hCG) is homologous to the alpha subunit of TSH. Thus, hCG in high concentrations has mild thyroid-stimulating activity.
The serum hCG concentration is highest in the first trimester of pregnancy and hCG’s thyroid-stimulating activity can suppress the serum TSH level, but in most cases the TSH level remains within the “normal range” of pregnancy.21,22 The hCG levels observed during the first trimester of pregnancy are usually associated with a low TSH and normal free thyroid hormone levels. In pregnant women who are not on T4 therapy for hypothyroidism, a persistently suppressed TSH (< 0.1 μIU/mL) after the first trimester or elevations of the free thyroid hormones at any point during pregnancy suggest that the suppressed TSH is secondary to autonomous thyroid function, as seen in Graves disease and toxic nodular goiters, warranting further investigation. Iodine radioisotope imaging studies are forbidden during pregnancy.
If the hCG concentration is markedly elevated and for a prolonged time, as in hyperemesis gravidarum and gestational trophoblastic disease (hydatidiform mole, a benign condition, and choriocarcinoma, a malignant condition), overt hyperthyroidism can develop, with elevated free T4 and free T3.21,23
LOW TSH, NORMAL FREE T4, LOW FREE T3
Euthyroid sick syndrome and/or medication effect. When the TSH level is low secondary to euthyroid sick syndrome or a drug, or both, the free T3 level is usually found to be also low, which may be solely related to a component of euthyroid sick syndrome or secondary to the drugs themselves, as drugs such as corticosteroids and amiodarone inhibit the conversion of T4 to T3.
LOW TSH, NORMAL FREE T4, HIGH FREE T3
Toxic nodular goiter vs early Graves disease
If the free T3 is elevated and the TSH is low (suppressed), even in the absence of symptoms, a diagnosis of subclinical hyperthyroidism would be inappropriate, because by definition the free T4 and free T3 levels must be normal for a diagnosis of subclinical hyperthyroidism. The diagnostic possibilities are toxic nodular goiter and early Graves disease.
The combination of high T3, suppressed TSH, and normal T4 is usually associated with toxic nodular goiter, whereas T3 and T4 are typically both elevated in Graves disease (although T3 is usually more elevated than T4).24
The patient should also be tested for TSH receptor antibodies (TRAB), both stimulating and blocking, which are very specific for Graves disease.
Natural thyroid preparations
Natural thyroid preparations, which can contain large amounts of T3, can also yield the combination of normal free T4 and high free T3. Since these preparations contain both T4 and T3, they usually result in low TSH, normal free T4, and elevated free T3 levels when given in supratherapeutic doses. However, if these preparations are consumed in large enough quantities, both the free T4 and free T3 can be elevated. This is in contrast to supratherapeutic monotherapy with T3 (liothyronine), which usually results in low TSH, low free T4, and high free T3.
LOW TSH, HIGH FREE T4, NORMAL OR HIGH FREE T3
If the free T4 level is high, the free T3 level is usually high as well. Patients should undergo iodine 123 nuclear imaging.
If iodine 123 uptake is high
Graves disease vs toxic nodular goiter. If iodine 123 uptake is high, a low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones, is consistent with overt hyperthyroidism secondary to autonomous (TSH-independent) thyroid function.
Graves patients usually test positive for TRAB, and they may have related ophthalmopathy, whereas patients with toxic nodular goiter are TRAB-negative and do not have Graves ophthalmopathy.24–27
Patients with either Graves disease or toxic nodular goiter have increased iodine 123 uptake; however, the pattern of uptake in Graves disease is diffuse, whereas it is patchy or nodular when toxic nodular goiter is the underlying etiology (Figure 3).24,27 Complicating matters, the pattern of uptake in Graves disease may be patchy if the patient has been pretreated with antithyroid drugs such as propylthiouracil or methimazole (Tapazole).
Review of the patient’s history is important, as a recent iodine load (eg, intravenous contrast medium that contains iodine) can transiently worsen thyrotoxicosis while causing the iodine 123 uptake to be low. The reason for the low uptake is that the gland becomes saturated with “cold” (nonradiolabeled) iodine from the contrast medium and cannot take up more iodine (radiolabeled) for the radionuclide scan. For this reason, iodine 123 imaging should not be performed for 6 to 8 weeks after an exogenous load of iodine. In this circumstance, the history and physical examination, as well as laboratory testing (for TRAB), must be relied on to make the correct diagnosis.
Elevated human chorionic gonadotropin. Iodine 123 nuclear imaging studies are forbidden during pregnancy. Therefore, all women of childbearing age should have a pregnancy test before undergoing radioisotope imaging. If thyrotoxicosis from hCG is suspected (eg, in cases of hydatidiform mole or choriocarcinoma), ultrasonography of the uterus should be done to rule out a viable pregnancy before pursuing radioisotope imaging.
Treatment options for the usual causes of hyperthyroidism (toxic nodular goiter or Graves disease) include radioactive iodine ablation (unless the patient was exposed to a recent cold iodine load), antithyroid drugs (methimazole or propylthiouracil), or surgical resection (partial or complete thyroidectomy).27
Patients with overt hyperthyroidism should be referred to an endocrinologist for a thorough evaluation and discussion of the diagnosis and the treatments that are available. Beta-blockers can be used to ameliorate the symptoms of thyrotoxicosis such as palpitations, anxiety, and tremor.
If iodine 123 uptake is low
A low (suppressed) TSH level, in conjunction with elevations of the free thyroid hormones and low uptake of iodine 123, is consistent with overt hyperthyroidism secondary to:
- Thyroiditis
- Ectopic hyperthyroidism due to T4-T3 therapy, struma ovarii (very rare), or large deposits of functioning thyroid cancer metastases (very rare)
- Iodine-induced hyperthyroidism (Jod-Basedow effect)
- Amiodarone-induced thyrotoxicosis.27,28
Thyroiditis, ie, destruction or inflammation of thyroid tissue with subsequent release of preformed thyroid hormones into the circulation, results in thyrotoxicosis. The severity and duration of thyrotoxicosis depends not only on the size of the injured thyroid gland and the store of preformed thyroid hormones, but also on the extent and duration of the thyroid destruction and injury.
Subacute thyroiditis usually lasts several weeks to a few months, and typically follows a pattern of:
- Transient hyperthyroidism due to release of thyroid hormone stores
- A brief period of euthyroidism
- Hypothyroidism, as the store of preformed thyroid hormone is exhausted and thyroid inflammation and destruction subside, and then
- Recovery (usually, unless the thyroid is not capable of recovery), during which the TSH level rises in response to low levels of thyroid hormones in the circulation, and the recovering thyroid begins to resume thyroid hormone synthesis.28
There is a brief period during the hypothyroid phase of thyroiditis during which the TSH level remains low (or inappropriately normal), even though the free thyroid hormone levels are also low; this period is commonly called the “disequilibrium state” (Figure 2). This state is due to the slow recovery of the pituitary thyrotrophs as they escape tonic suppression by excess thyroid hormones.
The classic entity of de Quervain thyroiditis (subacute granulomatous thyroiditis) is painful, whereas other forms are painless (eg, autoimmune lymphocytic thyroiditis, postpartum, or related to cytokine [interferon] or lithium therapy).28 Other forms of thyroiditis, which may or may not be painful, include those induced by amiodarone, radiation, or trauma.
Regardless of the cause, watchful waiting is warranted while monitoring thyroid function tests to ensure that recovery takes place.28 Beta-blockers are often used to decrease symptoms during the transient hyperthyroid state observed early in the course of thyroiditis.
Ectopic hyperthyroidism. Ingestion of exogenous T4, T3, or both can suppress thyroid function. This can occur with supratherapeutic T4 and T3 (usually for hypothyroidism) and also factitiously or in patients abusing the drugs to lose weight. A useful way to differentiate exogenous from endogenous causes of thyrotoxicosis is to measure serum thyroglobulin, which would be decreased in the former and elevated in the latter.
Ectopic production of T4 and T3 can occur, albeit rarely, as in cases of struma ovarii or in patients with large deposits of functioning thyroid cancer metastases.29–31 Struma ovarii is a very rare ovarian teratoma (accounting for 1% of all ovarian tumors), and even when present it does not usually result in thyrotoxicosis. 29,30 However, the diagnosis should be considered in the appropriate clinical context, ie, in the setting of thyrotoxicosis and a pelvic mass; radioiodine uptake would be elevated in the pelvis in those cases.
Likewise, thyrotoxicosis secondary to functioning thyroid cancer metastases is also rare but should be considered in the right clinical context (iodine-avid tissue throughout the body noted on radioiodine whole-body imaging).
Iodine-induced hyperthyroidism develops in patients with underlying thyroid disease (toxic nodular goiter or Graves disease) and is caused by an exacerbation of autonomous (TSH-independent) thyroid function by an iodine load (eg, intravenous contrast medium that contains iodine, or amiodarone therapy [see below]).
Amiodarone-induced thyrotoxicosis. In various reports, the incidence of amiodaroneinduced thyrotoxicosis ranged from 1% to 23%.32 There are two types.
Type 1 is a form of iodine-induced hyperthyroidism. It can occur in patients with autonomous thyroid function when they are exposed to amiodarone, which contains 37% iodine by weight.
Type 2 occurs in patients with no underlying thyroid disease and is probably a consequence of a drug-induced destructive thyroiditis. Mixed or indeterminate forms of amiodarone-induced thyrotoxicosis encompassing several features of both type 1 and type 2 may also be observed.20
The treatment varies by type: antithyroid drugs (thionamides) in type 1 and corticosteroids in type 2.20 It can be difficult to discern between the two entities, and combination therapy with antithyroid drugs and prednisone may be needed. One of the drugs is then withdrawn, and the effect on the levels of free thyroid hormones is monitored. This helps determine which drug is working, pointing to the correct diagnosis and treatment.
CASE CONCLUDED
Our patient’s thyroid function tests were repeated at the time of her endocrinology consult, 2 weeks after she was noted to have a low TSH in the setting of low free T4, which suggested central hypothyroidism. Her TSH level was now 3.5 μIU/mL, and her free T4 level was 0.8. Thus, her low TSH in the setting of the low free T4 noted 2 weeks earlier reflected a disequilibrium state, which occurs during the hypothyroid phase of thyroiditis (Figure 2).
Repeated measurements of her thyroid function tests verified complete recovery and resolution of her thyroiditis. No levothyroxine therapy was required, and no further investigation was performed.
Acknowledgments: We thank Nada Johnson from the Department of Endocrinology, Cleveland Clinic, for her skillful help with the preparation of the figures.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54:300–306.
- Franklyn JA, Black EG, Betteridge J, Sheppard MC. Comparison of second and third generation methods for measurement of serum thyrotropin in patients with overt hyperthyroidism, patients receiving thyroxine therapy, and those with nonthyroidal illness. J Clin Endocrinol Metab 1994; 78:1368–1371.
- Rosario PW. The natural history of subclinical hyperthyroidism in patients below the age of 65 years. Clin Endocrinol (Oxf) 2008; 68:491–492.
- Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/L: a prospective study. Clin Endocrinol (Oxf) 2009 Sep 10. [Epub ahead of print]
- Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid 2005; 15:687–691.
- Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med 1995; 46:197–204.
- Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007; 92:3–9.
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33:1391–1396.
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48:2096–2103.
- Brabant A, Brabant G, Schuermeyer T, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol (Copenh) 1989; 121:95–100.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996; 17:610–638.
- Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989; 50:44–50.
- Murray RD, Kim K, Ren SG, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004; 89:3027–3032.
- Lightman SL, Fox P, Dunne MJ. The effect of SMS 201–995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl 1986; 119:84–95.
- Nicoloff JT, Fisher DA, Appleman MD. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49:1922–1929.
- Kirkegaard C, Nørgaard K, Snorgaard O, Bek T, Larsen M, Lund-Andersen H. Effect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in type I (insulin-dependent) diabetes mellitus. Acta Endocrinol (Copenh) 1990; 122:766–772.
- Colao A, Merola B, Ferone D, et al. Acute and chronic effects of octreotide on thyroid axis in growth hormone-secreting and clinically nonfunctioning pituitary adenomas. Eur J Endocrinol 1995; 133:189–194.
- Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980; 51:387–393.
- Lee E, Chen P, Rao H, Lee J, Burmeister LA. Effect of acute high dose dobutamine administration on serum thyrotrophin (TSH). Clin Endocrinol (Oxf) 1999; 50:487–492.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 2001; 22:240–254.
- Fantz CR, Dagogo-Jack S, Ladenson JH, Gronowski AM. Thyroid function during pregnancy. Clin Chem 1999; 45:2250–2258.
- Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999; 9:653–657.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med 2008; 358:2594–2605.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010; 362:726–738.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009; 360:994–1001.
- Cooper DS. Hyperthyroidism. Lancet 2003; 362:459–468.
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998; 27:169–185.
- Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet 1993; 42:143–146.
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993; 17:1210–1224.
- Kasagi K, Takeuchi R, Miyamoto S, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clin Endocrinol (Oxf) 1994; 40:429–434.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997; 126:63–73.
KEY POINTS
- A low TSH value should always be followed up by measuring the thyroid hormones, ie, thyroxine (T4) and triiodothyronine (T3).
- Serum levels of free thyroid hormones should be used when interpreting an abnormal TSH level, especially in the acute and inpatient settings.
- A low TSH level is not always the result of suppression by elevations in circulating thyroid hormones.
- A low TSH level in the setting of normal levels of free thyroid hormones should always be reassessed in 4 to 6 weeks before making a diagnosis.
- Overt hyperthyroidism is usually associated with a frankly suppressed TSH (< 0.1 μIU/mL).