User login
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
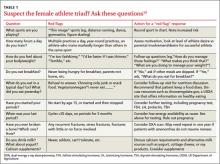
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.