User login
Does your patient really need testosterone replacement?
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
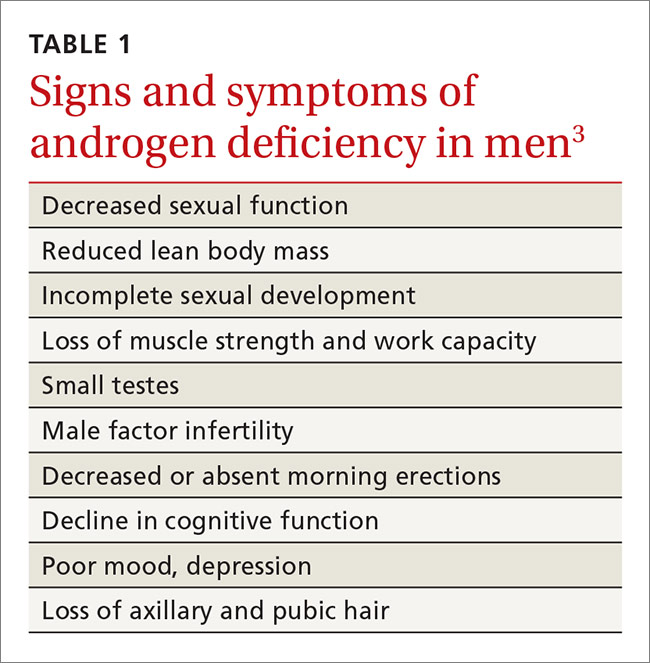
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
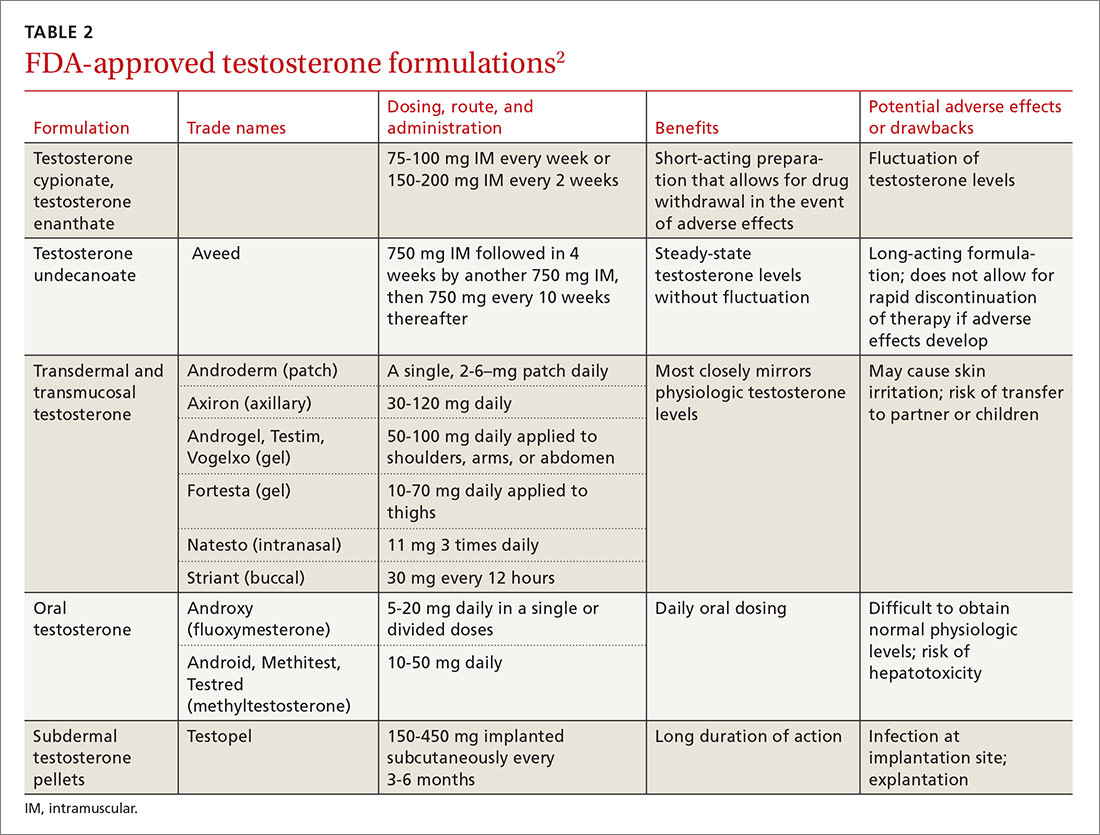
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
PRACTICE RECOMMENDATIONS
› Confirm suspected hypogonadism by getting 2 serum testosterone levels at least one month apart prior to initiating testosterone replacement therapy. B
› Consider testosterone replacement therapy when there is both laboratory and clinical evidence of hypogonadism. B
› Offer testosterone replacement to older men (≥65 years) with hypogonadism only after talking to them about the risks and benefits. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
HIV: 3 cases that hid in plain sight
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
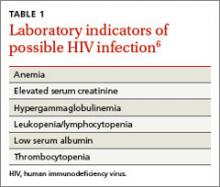
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
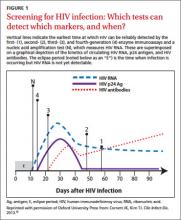
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; jtkirchn@lghealth.org
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; jtkirchn@lghealth.org
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; jtkirchn@lghealth.org
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
HIV screening: How we can do better
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
Should you suspect the female athlete triad?
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; jpayne2@lghealth.org
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.