User login
Does your patient really need testosterone replacement?
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
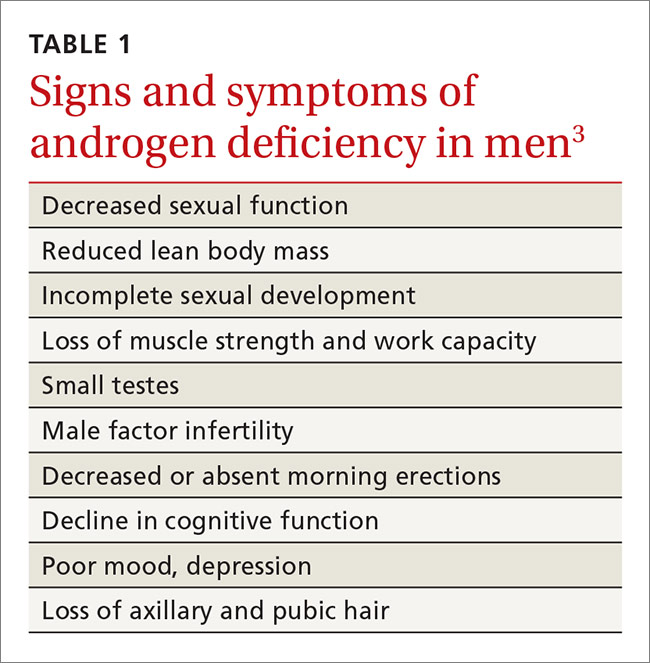
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
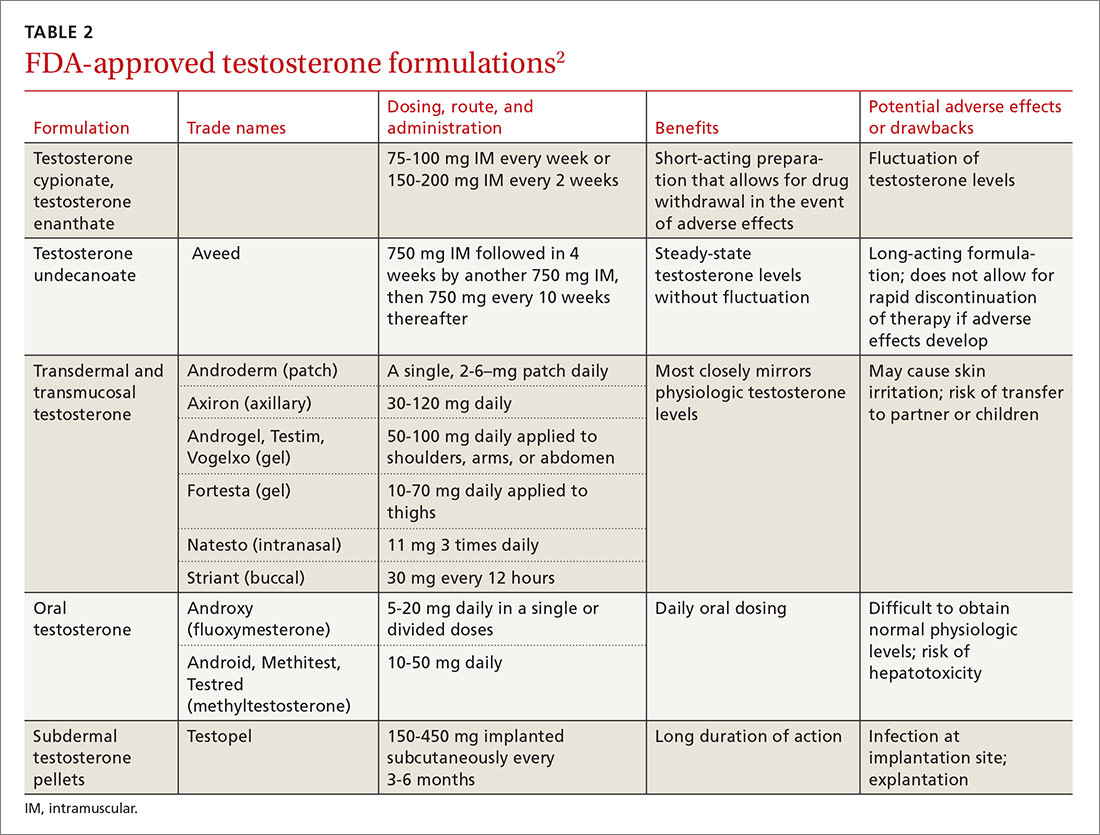
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; jhoover4@lghealth.org.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
PRACTICE RECOMMENDATIONS
› Confirm suspected hypogonadism by getting 2 serum testosterone levels at least one month apart prior to initiating testosterone replacement therapy. B
› Consider testosterone replacement therapy when there is both laboratory and clinical evidence of hypogonadism. B
› Offer testosterone replacement to older men (≥65 years) with hypogonadism only after talking to them about the risks and benefits. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series