User login
Cyclophosphamide, an agent used to treat various malignant and autoimmune disorders, can cause severe hyponatremia with seizures in rare cases. The exact mechanism of cyclophosphamide-induced hyponatremia is poorly understood, but is thought to occur from a drug- associated antidiuretic hormone (ADH) release leading to free water retention.1 This unusual phenomenon of cyclophosphamide-associated syndrome of inappropriate antidiuretic hormone secretion (SIADH) has been described only in case reports, most of which reported the development of severe hyponatremia within a week after administration of cyclophosphamide.2-5 We report a unique case of a patient who developed severe, symptomatic hyponatremia with seizures, with her serum sodium decreasing from 137 mEq to 112 mEq within 30 hours after her first dose of low-dose cyclophosphamide (600 mg/m2).
Case presentation and summary
A 68-year-old white woman with a history of bilateral invasive ductal carcinoma of the breast (status-post bilateral mastectomy) presented to the emergency department (ED) at our facility with new onset seizure. The patient had been diagnosed 8 months earlier with stage I (T1c, N0, M0) poorly differentiated infiltrating ductal carcinoma (triple negative) of the left breast for which she underwent left segmental mastectomy about 1 month after diagnosis. She was subsequently found to have progressive disease with stage IIIC (T2, N3, and M0) infiltrating ductal carcinoma with lobular features (ER/PR+, Her2) of the right breast. She underwent a right modified radical mastectomy 5 months after her stage IIIC breast cancer diagnosis. She received her first cycle of adjuvant chemotherapy with intravenous doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), which included pre-hydration, a day before presenting to our facility.
According to the patient’s family who provided the initial history, the patient reported tightness in her left arm while sitting at the dinner table. She was confused and subsequently had jerking movement of her right upper extremity with left facial twitching which lasted about 40 seconds. There was no loss of consciousness, or bowel or bladder control. She became unresponsive after the episode. Review of systems was negative except for a report of nausea a few hours before the onset of seizures, which resolved with ondansetron. Her past medical history was significant for breast cancer as already mentioned, seasonal allergic rhinitis, and hypertension. Home medications included hydrochlorothiazide 12.5 mg oral daily, aspirin 81 mg oral daily, and fexofenadine and loratadine oral daily as needed for allergies. There were no other significant surgical history other than already stated. The patient lived at home with her family and was independent with her instrumental activities of daily living. She is a former smoker of tobacco and quit smoking 30 years ago.
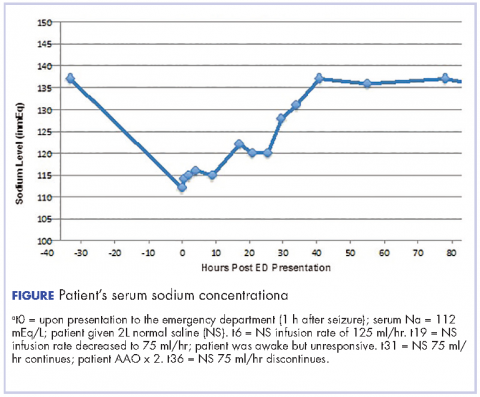
On arrival at our facility, the patient had normal vital signs. Significant findings on physical examination were an elderly female who seemed somnolent; not able to follow commands with a documented Glasgow Coma Scale of 10 with eyes opening spontaneously, incomprehensible sounds, and flexion withdrawal from pain as her best responses. She had an increased tone in her left upper extremity and had a brisk, deep tendon reflexes without clonus or 3+ (range, 0-5+, with 2+ being normal). The remainder of her physical exam was unremarkable. Laboratory testing revealed a glucose level of 120 mg/dL (normal, 65-110 mg/dL), sodium of 112 mEq/L (normal, 135-145 mmol/L), and chloride of 78 mEq/L (normal, 95-105 mmol/L). Serum osmolality and urine osmolality were 242 mOsm/kg (normal, 282-295 mOsm/kg) and 449 mOsm/kg (normal, 500-800 mOsm/kg) respectively, indicative of suboptimally dilute urine despite relatively low serum osmolality or SIADH. Urine electrolytes were not obtained.
Imaging studies including computed-tomography scans of the head and chest x-ray performed in the ED were unremarkable. After a phenytoin load, an electroencephalogram was obtained which showed diffuse encephalopathy without active seizure foci. A non-contrast magnetic-resonance imaging (MRI) of the brain was performed but it failed to show acute infarct, mass, mass effect, or brain herniation. There was nonspecific white matter abnormality with compromise of the bilateral cerebral hemispheres, calloseptal junction, left posterior pillar, and bilateral anterior pillars of the fornix, possibly representative of chronic white matter microvascular ischemic changes or less likely vasculitis or demyelination. Correction of her hyponatremia with normal saline was started in the ED with a change in serum sodium from 112 mEq/L to 115 mEq/L within 2 hours. She was admitted to the intensive care unit (ICU) where her sodium correction with normal saline and free water restriction was continued with a goal correction rate of 8-12 mEq/L in 24 hours. The patient’s serum sodium as well as level of consciousness improved gradually over the course of her ICU stay. After 64 hours in the hospital, her sodium had corrected to 137 mEq/L (normal, 135-145 mmol/L; Figure). She was then alert and oriented to person, place, and time. All motor findings noted on presentation had resolved. Her saline infusion was discontinued and serum sodium remained within normal range. She was discharged to a rehabilitation facility. Her hydrochlorothiazide was also discontinued.
Discussion
Hyponatremia is a common finding in cancer patients caused usually by paraneoplastic syndrome, chemotherapy, immunotherapy, or other associated treatment.6 SIADH is a frequent cause of hyponatremia in cancer patients and should be suspected in patients with hyponatremia, hypo-osmolality, and a urine osmolality above 100 mOsmol/kg.7
Our patient’s presentation and laboratory findings suggested SIADH as the likely cause of hyponatremia with a low sodium, a serum osmolality 242 mOsm/kg and urine osmolality of 449 mOsm/kg.8-10 She had no known underlying contributory comorbid condition relating to her serum lipids, thyroid, adrenal, kidney, or heart to date. Her use of a thiazide diuretic was the only confounding factor. The most plausible cause of hyponatremia/SIADH in our patient was likely cyclophosphamide based on her history, timeline of symptoms, and the absence of other possible causes. Though the mechanism for many of the previously mentioned etiologies are known, the mechanism of cyclophosphamide-induced SIADH is difficult to elucidate since the imminent complication of hemorrhagic cystitis means patients receiving this drug are often aggressively hydrated to prevent this complication.11,12 The result is that there is marked retention of water leading to potentially fatal hyponatremia in selected cases.11 This phenomenon has been fairly well described in patients receiving doses of 6 g/m2 as given in the STAMP protocol for stem cell mobilization or at doses of 30-50 mg/kg used to treat malignancy.12 Our patient clearly falls in this category given that she received a dose of 600 mg/m2. We found no evidence in her history to suggest post-operative, genetic or other cause for her hyponatremia. Our case mirrors a report by Koo and colleagues who described severe hyponatremia occurring within 24 hours following a single dose of intravenous cyclophosphamide 700 mg followed by saline infusion.13 In the case reported by Jayachandra and colleagues in which suspected cyclophosphamide-induced hyponatremia led to seizures, the patient received 500 mg IV of cyclophosphamide and had serum sodium as low as 106 mEq/L within a 24-hour period,2 similar to our patient.
There is a paucity of data on cyclophosphamide-induced SIADH. The mechanism by which cyclophosphamide causes SIADH is currently unknown. In addition, there are currently no set criteria that help identify at-risk patients who may develop such an event, including the dosage of cyclophosphamide that may trigger the SIADH, because lower doses of the drug have been associated with this complication.14
In a retrospective analysis by Lee and colleagues, cyclophosphamide-induced hyponatremia was found to be associated with male sex on a univariate analysis, but no risk factors were found in a multivariate analysis.15 It is likely that the concomitant use of diuretics, hydration, and high-dose cyclophosphamide contributed to hyponatremia/SIADH in our patient, though it is not clear through what mechanism. Harlow and colleagues proposed a mechanism for this phenomenon in 1979 based on the autopsy of a patient who had received high-dose cyclophosphamide involving degranulation of hypothalamic neurosecretory organelles and loss of Herring’s bodies. They inferred that metabolites of cyclophosphamide indirectly triggered inappropriate secretion of antidiuretic hormone as seen with a use of the structurally related analogue ifosfamide,16 but to our knowledge, this has yet to be replicated. Cyclophosphamide metabolite may have a direct tubular effect on the collecting duct epithelium leading to water retention15 as established by Campbell and colleagues. In one case, an established diabetes insipidus patient developed cyclophosphamide-induced antidiuresis without vasopressin secretion.17 It is imperative that the scientific community conduct research into the risk factors, underlying mechanisms, and methods of prevention to reduce and/or eliminate SIADH associated with use of cyclophosphamide.
1. Gilbar PJ, Richmond J, Wood J, Sullivan A. Syndrome of inappropriate antidiuretic hormone secretion induced by a single dose of oral cyclophosphamide. Ann Pharmacother. 2012.46(9):e23.
2. Jayachandran NV, Chandrasekhara PK, Thomas J, Agrawal S, Narsimulu G. Cyclophosphamide-associated complications: we need to be aware of SIADH and central pontine myelinolysis. Rheumatology (Oxford). 2009;48(1):89-90.
3. Baker M, Markman M, Niu J. Cyclophosphamide-induced severe acute hyponatremic encephalopathy in patients with breast cancer: report of two cases. Case Rep Oncol. 2014;7(2):550-554.
4. Lazarevic V, Hägg E, Wahlin A. Hiccups and severe hyponatremia associated with high-dose cyclophosphamide in conditioning regimen for allogeneic stem cell transplantation. Am J Hematol. 2007;82(1):88.
5. Geng C, Tang P, Zhang Y, Gao W. Hyponatremia induced by low-dose cyclophosphamide in two patients with breast cancer. Breast J. 2014; 20(4):442-443.
6. Kamoi K, Ebe T, Hasegawa A, et al. Hyponatremia in small cell lung cancer. Mechanisms not involving inappropriate ADH secretion. Cancer. 1987;60(5):1089-1093.
7. Matwiejczuk S, Püsküllüoğlu M, Zygulska AL. Oncological emergencies: syndrome of inappropriate antidiuretic hormone secretion (SIADH). Przegl Lek. 2014;71(10):541-543.
8. Robertson GL. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 2006;119(7 Suppl 1):S36-42.
9. Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int. 1976;10(1):25-37.
10. Decaux G, Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3(4):1175-1184.
11. Bressler RB, Huston DP. Water intoxication following moderate-dose intravenous cyclophosphamide. Arch Intern Med. 1985;145(3):548-549.
12. Salido M, Macarron P, Hernández-García C, D’Cruz DP, Khamashta MA, Hughes GR. Water intoxication induced by low-dose cyclophosphamide in two patients with systemic lupus erythematosus. Lupus. 2003;12(8):636-639.
13. Koo TY, Bae SC, Park JS, et al. Water intoxication following low-dose intravenous cyclophosphamide. Electrolyte Blood Press. 2007;5(1):50-54.
14. [No authors listed]. Nausea and vasopressin. Lancet. 1991;337(8750):1133-1134.
15 Lee YC1, Park JS, Lee CH, et al. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25(5):1520-1524.
16. Harlow PJ, DeClerck YA, Shore NA, Ortega JA, Carranza A, Heuser E. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44(3):896-898.
17. Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000;13(6):673-675.
Cyclophosphamide, an agent used to treat various malignant and autoimmune disorders, can cause severe hyponatremia with seizures in rare cases. The exact mechanism of cyclophosphamide-induced hyponatremia is poorly understood, but is thought to occur from a drug- associated antidiuretic hormone (ADH) release leading to free water retention.1 This unusual phenomenon of cyclophosphamide-associated syndrome of inappropriate antidiuretic hormone secretion (SIADH) has been described only in case reports, most of which reported the development of severe hyponatremia within a week after administration of cyclophosphamide.2-5 We report a unique case of a patient who developed severe, symptomatic hyponatremia with seizures, with her serum sodium decreasing from 137 mEq to 112 mEq within 30 hours after her first dose of low-dose cyclophosphamide (600 mg/m2).
Case presentation and summary
A 68-year-old white woman with a history of bilateral invasive ductal carcinoma of the breast (status-post bilateral mastectomy) presented to the emergency department (ED) at our facility with new onset seizure. The patient had been diagnosed 8 months earlier with stage I (T1c, N0, M0) poorly differentiated infiltrating ductal carcinoma (triple negative) of the left breast for which she underwent left segmental mastectomy about 1 month after diagnosis. She was subsequently found to have progressive disease with stage IIIC (T2, N3, and M0) infiltrating ductal carcinoma with lobular features (ER/PR+, Her2) of the right breast. She underwent a right modified radical mastectomy 5 months after her stage IIIC breast cancer diagnosis. She received her first cycle of adjuvant chemotherapy with intravenous doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), which included pre-hydration, a day before presenting to our facility.
According to the patient’s family who provided the initial history, the patient reported tightness in her left arm while sitting at the dinner table. She was confused and subsequently had jerking movement of her right upper extremity with left facial twitching which lasted about 40 seconds. There was no loss of consciousness, or bowel or bladder control. She became unresponsive after the episode. Review of systems was negative except for a report of nausea a few hours before the onset of seizures, which resolved with ondansetron. Her past medical history was significant for breast cancer as already mentioned, seasonal allergic rhinitis, and hypertension. Home medications included hydrochlorothiazide 12.5 mg oral daily, aspirin 81 mg oral daily, and fexofenadine and loratadine oral daily as needed for allergies. There were no other significant surgical history other than already stated. The patient lived at home with her family and was independent with her instrumental activities of daily living. She is a former smoker of tobacco and quit smoking 30 years ago.
On arrival at our facility, the patient had normal vital signs. Significant findings on physical examination were an elderly female who seemed somnolent; not able to follow commands with a documented Glasgow Coma Scale of 10 with eyes opening spontaneously, incomprehensible sounds, and flexion withdrawal from pain as her best responses. She had an increased tone in her left upper extremity and had a brisk, deep tendon reflexes without clonus or 3+ (range, 0-5+, with 2+ being normal). The remainder of her physical exam was unremarkable. Laboratory testing revealed a glucose level of 120 mg/dL (normal, 65-110 mg/dL), sodium of 112 mEq/L (normal, 135-145 mmol/L), and chloride of 78 mEq/L (normal, 95-105 mmol/L). Serum osmolality and urine osmolality were 242 mOsm/kg (normal, 282-295 mOsm/kg) and 449 mOsm/kg (normal, 500-800 mOsm/kg) respectively, indicative of suboptimally dilute urine despite relatively low serum osmolality or SIADH. Urine electrolytes were not obtained.
Imaging studies including computed-tomography scans of the head and chest x-ray performed in the ED were unremarkable. After a phenytoin load, an electroencephalogram was obtained which showed diffuse encephalopathy without active seizure foci. A non-contrast magnetic-resonance imaging (MRI) of the brain was performed but it failed to show acute infarct, mass, mass effect, or brain herniation. There was nonspecific white matter abnormality with compromise of the bilateral cerebral hemispheres, calloseptal junction, left posterior pillar, and bilateral anterior pillars of the fornix, possibly representative of chronic white matter microvascular ischemic changes or less likely vasculitis or demyelination. Correction of her hyponatremia with normal saline was started in the ED with a change in serum sodium from 112 mEq/L to 115 mEq/L within 2 hours. She was admitted to the intensive care unit (ICU) where her sodium correction with normal saline and free water restriction was continued with a goal correction rate of 8-12 mEq/L in 24 hours. The patient’s serum sodium as well as level of consciousness improved gradually over the course of her ICU stay. After 64 hours in the hospital, her sodium had corrected to 137 mEq/L (normal, 135-145 mmol/L; Figure). She was then alert and oriented to person, place, and time. All motor findings noted on presentation had resolved. Her saline infusion was discontinued and serum sodium remained within normal range. She was discharged to a rehabilitation facility. Her hydrochlorothiazide was also discontinued.
Discussion
Hyponatremia is a common finding in cancer patients caused usually by paraneoplastic syndrome, chemotherapy, immunotherapy, or other associated treatment.6 SIADH is a frequent cause of hyponatremia in cancer patients and should be suspected in patients with hyponatremia, hypo-osmolality, and a urine osmolality above 100 mOsmol/kg.7
Our patient’s presentation and laboratory findings suggested SIADH as the likely cause of hyponatremia with a low sodium, a serum osmolality 242 mOsm/kg and urine osmolality of 449 mOsm/kg.8-10 She had no known underlying contributory comorbid condition relating to her serum lipids, thyroid, adrenal, kidney, or heart to date. Her use of a thiazide diuretic was the only confounding factor. The most plausible cause of hyponatremia/SIADH in our patient was likely cyclophosphamide based on her history, timeline of symptoms, and the absence of other possible causes. Though the mechanism for many of the previously mentioned etiologies are known, the mechanism of cyclophosphamide-induced SIADH is difficult to elucidate since the imminent complication of hemorrhagic cystitis means patients receiving this drug are often aggressively hydrated to prevent this complication.11,12 The result is that there is marked retention of water leading to potentially fatal hyponatremia in selected cases.11 This phenomenon has been fairly well described in patients receiving doses of 6 g/m2 as given in the STAMP protocol for stem cell mobilization or at doses of 30-50 mg/kg used to treat malignancy.12 Our patient clearly falls in this category given that she received a dose of 600 mg/m2. We found no evidence in her history to suggest post-operative, genetic or other cause for her hyponatremia. Our case mirrors a report by Koo and colleagues who described severe hyponatremia occurring within 24 hours following a single dose of intravenous cyclophosphamide 700 mg followed by saline infusion.13 In the case reported by Jayachandra and colleagues in which suspected cyclophosphamide-induced hyponatremia led to seizures, the patient received 500 mg IV of cyclophosphamide and had serum sodium as low as 106 mEq/L within a 24-hour period,2 similar to our patient.
There is a paucity of data on cyclophosphamide-induced SIADH. The mechanism by which cyclophosphamide causes SIADH is currently unknown. In addition, there are currently no set criteria that help identify at-risk patients who may develop such an event, including the dosage of cyclophosphamide that may trigger the SIADH, because lower doses of the drug have been associated with this complication.14
In a retrospective analysis by Lee and colleagues, cyclophosphamide-induced hyponatremia was found to be associated with male sex on a univariate analysis, but no risk factors were found in a multivariate analysis.15 It is likely that the concomitant use of diuretics, hydration, and high-dose cyclophosphamide contributed to hyponatremia/SIADH in our patient, though it is not clear through what mechanism. Harlow and colleagues proposed a mechanism for this phenomenon in 1979 based on the autopsy of a patient who had received high-dose cyclophosphamide involving degranulation of hypothalamic neurosecretory organelles and loss of Herring’s bodies. They inferred that metabolites of cyclophosphamide indirectly triggered inappropriate secretion of antidiuretic hormone as seen with a use of the structurally related analogue ifosfamide,16 but to our knowledge, this has yet to be replicated. Cyclophosphamide metabolite may have a direct tubular effect on the collecting duct epithelium leading to water retention15 as established by Campbell and colleagues. In one case, an established diabetes insipidus patient developed cyclophosphamide-induced antidiuresis without vasopressin secretion.17 It is imperative that the scientific community conduct research into the risk factors, underlying mechanisms, and methods of prevention to reduce and/or eliminate SIADH associated with use of cyclophosphamide.
Cyclophosphamide, an agent used to treat various malignant and autoimmune disorders, can cause severe hyponatremia with seizures in rare cases. The exact mechanism of cyclophosphamide-induced hyponatremia is poorly understood, but is thought to occur from a drug- associated antidiuretic hormone (ADH) release leading to free water retention.1 This unusual phenomenon of cyclophosphamide-associated syndrome of inappropriate antidiuretic hormone secretion (SIADH) has been described only in case reports, most of which reported the development of severe hyponatremia within a week after administration of cyclophosphamide.2-5 We report a unique case of a patient who developed severe, symptomatic hyponatremia with seizures, with her serum sodium decreasing from 137 mEq to 112 mEq within 30 hours after her first dose of low-dose cyclophosphamide (600 mg/m2).
Case presentation and summary
A 68-year-old white woman with a history of bilateral invasive ductal carcinoma of the breast (status-post bilateral mastectomy) presented to the emergency department (ED) at our facility with new onset seizure. The patient had been diagnosed 8 months earlier with stage I (T1c, N0, M0) poorly differentiated infiltrating ductal carcinoma (triple negative) of the left breast for which she underwent left segmental mastectomy about 1 month after diagnosis. She was subsequently found to have progressive disease with stage IIIC (T2, N3, and M0) infiltrating ductal carcinoma with lobular features (ER/PR+, Her2) of the right breast. She underwent a right modified radical mastectomy 5 months after her stage IIIC breast cancer diagnosis. She received her first cycle of adjuvant chemotherapy with intravenous doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), which included pre-hydration, a day before presenting to our facility.
According to the patient’s family who provided the initial history, the patient reported tightness in her left arm while sitting at the dinner table. She was confused and subsequently had jerking movement of her right upper extremity with left facial twitching which lasted about 40 seconds. There was no loss of consciousness, or bowel or bladder control. She became unresponsive after the episode. Review of systems was negative except for a report of nausea a few hours before the onset of seizures, which resolved with ondansetron. Her past medical history was significant for breast cancer as already mentioned, seasonal allergic rhinitis, and hypertension. Home medications included hydrochlorothiazide 12.5 mg oral daily, aspirin 81 mg oral daily, and fexofenadine and loratadine oral daily as needed for allergies. There were no other significant surgical history other than already stated. The patient lived at home with her family and was independent with her instrumental activities of daily living. She is a former smoker of tobacco and quit smoking 30 years ago.
On arrival at our facility, the patient had normal vital signs. Significant findings on physical examination were an elderly female who seemed somnolent; not able to follow commands with a documented Glasgow Coma Scale of 10 with eyes opening spontaneously, incomprehensible sounds, and flexion withdrawal from pain as her best responses. She had an increased tone in her left upper extremity and had a brisk, deep tendon reflexes without clonus or 3+ (range, 0-5+, with 2+ being normal). The remainder of her physical exam was unremarkable. Laboratory testing revealed a glucose level of 120 mg/dL (normal, 65-110 mg/dL), sodium of 112 mEq/L (normal, 135-145 mmol/L), and chloride of 78 mEq/L (normal, 95-105 mmol/L). Serum osmolality and urine osmolality were 242 mOsm/kg (normal, 282-295 mOsm/kg) and 449 mOsm/kg (normal, 500-800 mOsm/kg) respectively, indicative of suboptimally dilute urine despite relatively low serum osmolality or SIADH. Urine electrolytes were not obtained.
Imaging studies including computed-tomography scans of the head and chest x-ray performed in the ED were unremarkable. After a phenytoin load, an electroencephalogram was obtained which showed diffuse encephalopathy without active seizure foci. A non-contrast magnetic-resonance imaging (MRI) of the brain was performed but it failed to show acute infarct, mass, mass effect, or brain herniation. There was nonspecific white matter abnormality with compromise of the bilateral cerebral hemispheres, calloseptal junction, left posterior pillar, and bilateral anterior pillars of the fornix, possibly representative of chronic white matter microvascular ischemic changes or less likely vasculitis or demyelination. Correction of her hyponatremia with normal saline was started in the ED with a change in serum sodium from 112 mEq/L to 115 mEq/L within 2 hours. She was admitted to the intensive care unit (ICU) where her sodium correction with normal saline and free water restriction was continued with a goal correction rate of 8-12 mEq/L in 24 hours. The patient’s serum sodium as well as level of consciousness improved gradually over the course of her ICU stay. After 64 hours in the hospital, her sodium had corrected to 137 mEq/L (normal, 135-145 mmol/L; Figure). She was then alert and oriented to person, place, and time. All motor findings noted on presentation had resolved. Her saline infusion was discontinued and serum sodium remained within normal range. She was discharged to a rehabilitation facility. Her hydrochlorothiazide was also discontinued.
Discussion
Hyponatremia is a common finding in cancer patients caused usually by paraneoplastic syndrome, chemotherapy, immunotherapy, or other associated treatment.6 SIADH is a frequent cause of hyponatremia in cancer patients and should be suspected in patients with hyponatremia, hypo-osmolality, and a urine osmolality above 100 mOsmol/kg.7
Our patient’s presentation and laboratory findings suggested SIADH as the likely cause of hyponatremia with a low sodium, a serum osmolality 242 mOsm/kg and urine osmolality of 449 mOsm/kg.8-10 She had no known underlying contributory comorbid condition relating to her serum lipids, thyroid, adrenal, kidney, or heart to date. Her use of a thiazide diuretic was the only confounding factor. The most plausible cause of hyponatremia/SIADH in our patient was likely cyclophosphamide based on her history, timeline of symptoms, and the absence of other possible causes. Though the mechanism for many of the previously mentioned etiologies are known, the mechanism of cyclophosphamide-induced SIADH is difficult to elucidate since the imminent complication of hemorrhagic cystitis means patients receiving this drug are often aggressively hydrated to prevent this complication.11,12 The result is that there is marked retention of water leading to potentially fatal hyponatremia in selected cases.11 This phenomenon has been fairly well described in patients receiving doses of 6 g/m2 as given in the STAMP protocol for stem cell mobilization or at doses of 30-50 mg/kg used to treat malignancy.12 Our patient clearly falls in this category given that she received a dose of 600 mg/m2. We found no evidence in her history to suggest post-operative, genetic or other cause for her hyponatremia. Our case mirrors a report by Koo and colleagues who described severe hyponatremia occurring within 24 hours following a single dose of intravenous cyclophosphamide 700 mg followed by saline infusion.13 In the case reported by Jayachandra and colleagues in which suspected cyclophosphamide-induced hyponatremia led to seizures, the patient received 500 mg IV of cyclophosphamide and had serum sodium as low as 106 mEq/L within a 24-hour period,2 similar to our patient.
There is a paucity of data on cyclophosphamide-induced SIADH. The mechanism by which cyclophosphamide causes SIADH is currently unknown. In addition, there are currently no set criteria that help identify at-risk patients who may develop such an event, including the dosage of cyclophosphamide that may trigger the SIADH, because lower doses of the drug have been associated with this complication.14
In a retrospective analysis by Lee and colleagues, cyclophosphamide-induced hyponatremia was found to be associated with male sex on a univariate analysis, but no risk factors were found in a multivariate analysis.15 It is likely that the concomitant use of diuretics, hydration, and high-dose cyclophosphamide contributed to hyponatremia/SIADH in our patient, though it is not clear through what mechanism. Harlow and colleagues proposed a mechanism for this phenomenon in 1979 based on the autopsy of a patient who had received high-dose cyclophosphamide involving degranulation of hypothalamic neurosecretory organelles and loss of Herring’s bodies. They inferred that metabolites of cyclophosphamide indirectly triggered inappropriate secretion of antidiuretic hormone as seen with a use of the structurally related analogue ifosfamide,16 but to our knowledge, this has yet to be replicated. Cyclophosphamide metabolite may have a direct tubular effect on the collecting duct epithelium leading to water retention15 as established by Campbell and colleagues. In one case, an established diabetes insipidus patient developed cyclophosphamide-induced antidiuresis without vasopressin secretion.17 It is imperative that the scientific community conduct research into the risk factors, underlying mechanisms, and methods of prevention to reduce and/or eliminate SIADH associated with use of cyclophosphamide.
1. Gilbar PJ, Richmond J, Wood J, Sullivan A. Syndrome of inappropriate antidiuretic hormone secretion induced by a single dose of oral cyclophosphamide. Ann Pharmacother. 2012.46(9):e23.
2. Jayachandran NV, Chandrasekhara PK, Thomas J, Agrawal S, Narsimulu G. Cyclophosphamide-associated complications: we need to be aware of SIADH and central pontine myelinolysis. Rheumatology (Oxford). 2009;48(1):89-90.
3. Baker M, Markman M, Niu J. Cyclophosphamide-induced severe acute hyponatremic encephalopathy in patients with breast cancer: report of two cases. Case Rep Oncol. 2014;7(2):550-554.
4. Lazarevic V, Hägg E, Wahlin A. Hiccups and severe hyponatremia associated with high-dose cyclophosphamide in conditioning regimen for allogeneic stem cell transplantation. Am J Hematol. 2007;82(1):88.
5. Geng C, Tang P, Zhang Y, Gao W. Hyponatremia induced by low-dose cyclophosphamide in two patients with breast cancer. Breast J. 2014; 20(4):442-443.
6. Kamoi K, Ebe T, Hasegawa A, et al. Hyponatremia in small cell lung cancer. Mechanisms not involving inappropriate ADH secretion. Cancer. 1987;60(5):1089-1093.
7. Matwiejczuk S, Püsküllüoğlu M, Zygulska AL. Oncological emergencies: syndrome of inappropriate antidiuretic hormone secretion (SIADH). Przegl Lek. 2014;71(10):541-543.
8. Robertson GL. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 2006;119(7 Suppl 1):S36-42.
9. Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int. 1976;10(1):25-37.
10. Decaux G, Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3(4):1175-1184.
11. Bressler RB, Huston DP. Water intoxication following moderate-dose intravenous cyclophosphamide. Arch Intern Med. 1985;145(3):548-549.
12. Salido M, Macarron P, Hernández-García C, D’Cruz DP, Khamashta MA, Hughes GR. Water intoxication induced by low-dose cyclophosphamide in two patients with systemic lupus erythematosus. Lupus. 2003;12(8):636-639.
13. Koo TY, Bae SC, Park JS, et al. Water intoxication following low-dose intravenous cyclophosphamide. Electrolyte Blood Press. 2007;5(1):50-54.
14. [No authors listed]. Nausea and vasopressin. Lancet. 1991;337(8750):1133-1134.
15 Lee YC1, Park JS, Lee CH, et al. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25(5):1520-1524.
16. Harlow PJ, DeClerck YA, Shore NA, Ortega JA, Carranza A, Heuser E. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44(3):896-898.
17. Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000;13(6):673-675.
1. Gilbar PJ, Richmond J, Wood J, Sullivan A. Syndrome of inappropriate antidiuretic hormone secretion induced by a single dose of oral cyclophosphamide. Ann Pharmacother. 2012.46(9):e23.
2. Jayachandran NV, Chandrasekhara PK, Thomas J, Agrawal S, Narsimulu G. Cyclophosphamide-associated complications: we need to be aware of SIADH and central pontine myelinolysis. Rheumatology (Oxford). 2009;48(1):89-90.
3. Baker M, Markman M, Niu J. Cyclophosphamide-induced severe acute hyponatremic encephalopathy in patients with breast cancer: report of two cases. Case Rep Oncol. 2014;7(2):550-554.
4. Lazarevic V, Hägg E, Wahlin A. Hiccups and severe hyponatremia associated with high-dose cyclophosphamide in conditioning regimen for allogeneic stem cell transplantation. Am J Hematol. 2007;82(1):88.
5. Geng C, Tang P, Zhang Y, Gao W. Hyponatremia induced by low-dose cyclophosphamide in two patients with breast cancer. Breast J. 2014; 20(4):442-443.
6. Kamoi K, Ebe T, Hasegawa A, et al. Hyponatremia in small cell lung cancer. Mechanisms not involving inappropriate ADH secretion. Cancer. 1987;60(5):1089-1093.
7. Matwiejczuk S, Püsküllüoğlu M, Zygulska AL. Oncological emergencies: syndrome of inappropriate antidiuretic hormone secretion (SIADH). Przegl Lek. 2014;71(10):541-543.
8. Robertson GL. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 2006;119(7 Suppl 1):S36-42.
9. Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int. 1976;10(1):25-37.
10. Decaux G, Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3(4):1175-1184.
11. Bressler RB, Huston DP. Water intoxication following moderate-dose intravenous cyclophosphamide. Arch Intern Med. 1985;145(3):548-549.
12. Salido M, Macarron P, Hernández-García C, D’Cruz DP, Khamashta MA, Hughes GR. Water intoxication induced by low-dose cyclophosphamide in two patients with systemic lupus erythematosus. Lupus. 2003;12(8):636-639.
13. Koo TY, Bae SC, Park JS, et al. Water intoxication following low-dose intravenous cyclophosphamide. Electrolyte Blood Press. 2007;5(1):50-54.
14. [No authors listed]. Nausea and vasopressin. Lancet. 1991;337(8750):1133-1134.
15 Lee YC1, Park JS, Lee CH, et al. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25(5):1520-1524.
16. Harlow PJ, DeClerck YA, Shore NA, Ortega JA, Carranza A, Heuser E. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44(3):896-898.
17. Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000;13(6):673-675.