User login
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
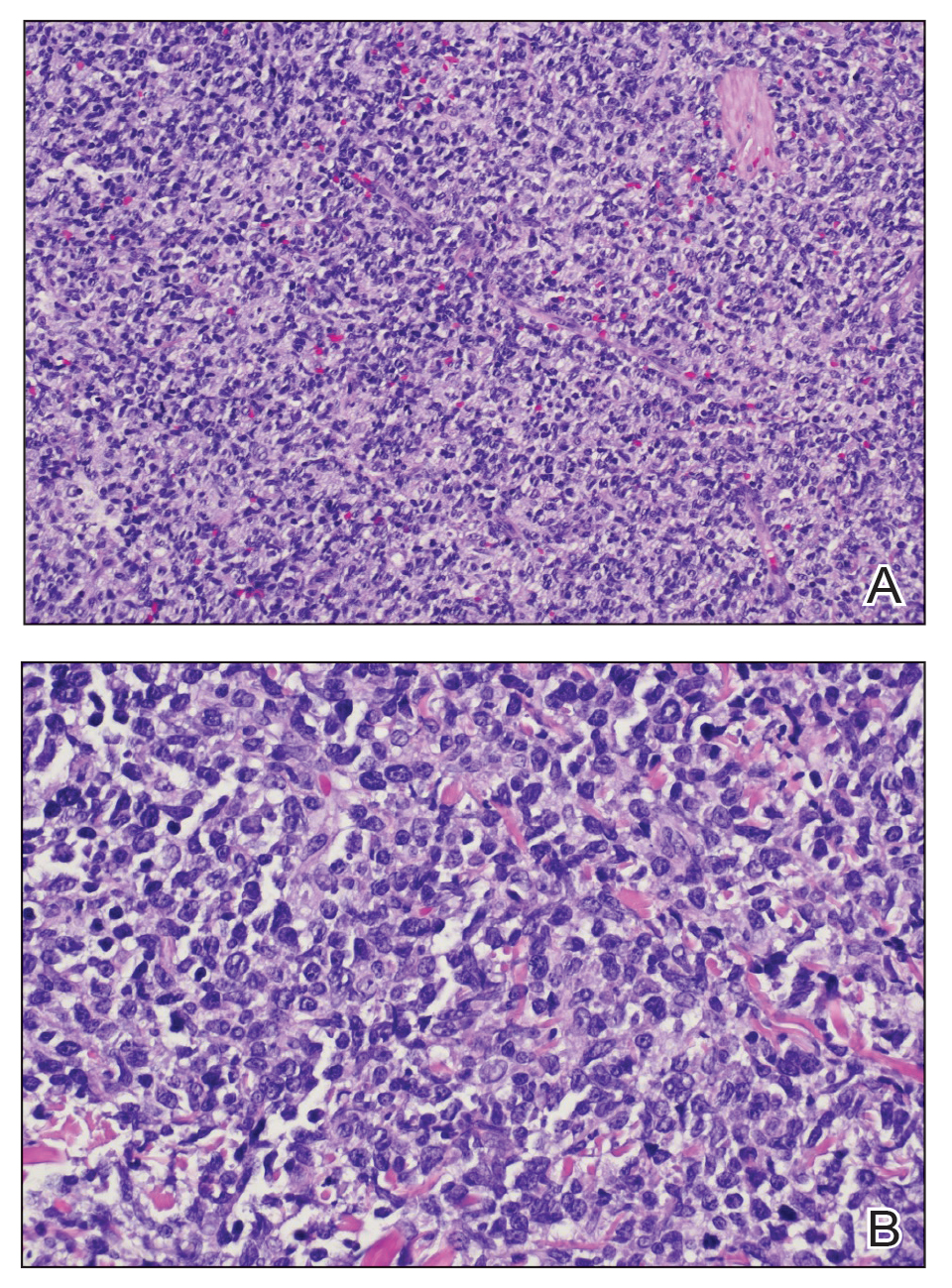
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
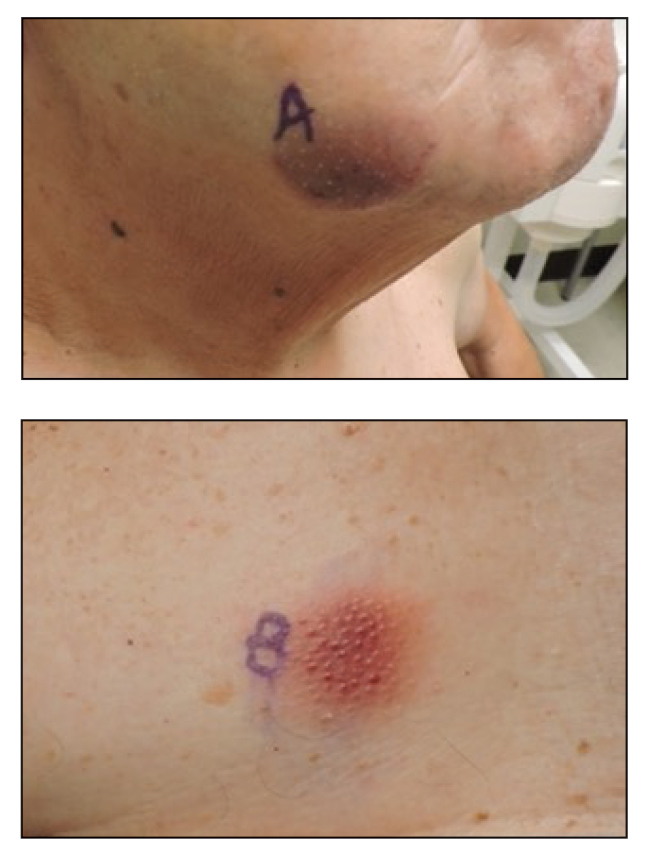
A 79-year-old man presented to the dermatology clinic with multiple skin lesions of 4 months’ duration. The patient had a history of monoclonal gammopathy and reported no changes in medication, travel, or trauma. He reported tenderness only when trying to comb hair over the left occipital nodule. He denied fevers, night sweats, weight loss, or poor appetite. Physical examination revealed 4 concerning skin lesions: a 3×3-cm violaceous nodule with underlying ecchymosis on the right medial jaw (top), a 3×2.5-cm violaceous nodule on the posterior occiput, a pink plaque with 1-mm vascular papules on the right mid-chest (bottom), and a 4×2.5-cm oval pink patch on the left side of the lower back. Punch biopsies were performed on the right medial jaw nodule and right mid-chest plaque.