User login
The Diagnosis: Acquired Cutaneous Lymphangiectasia
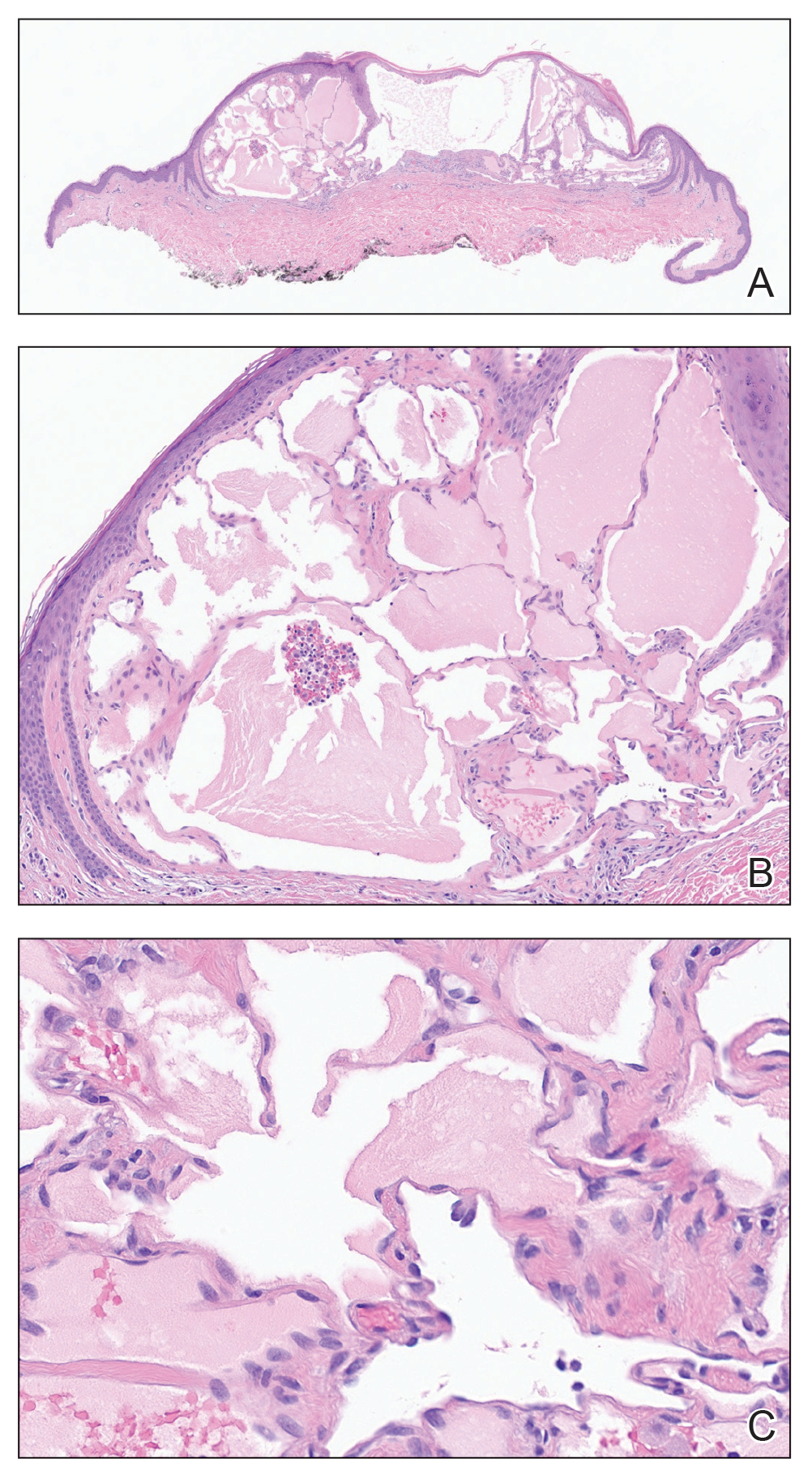
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.
Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
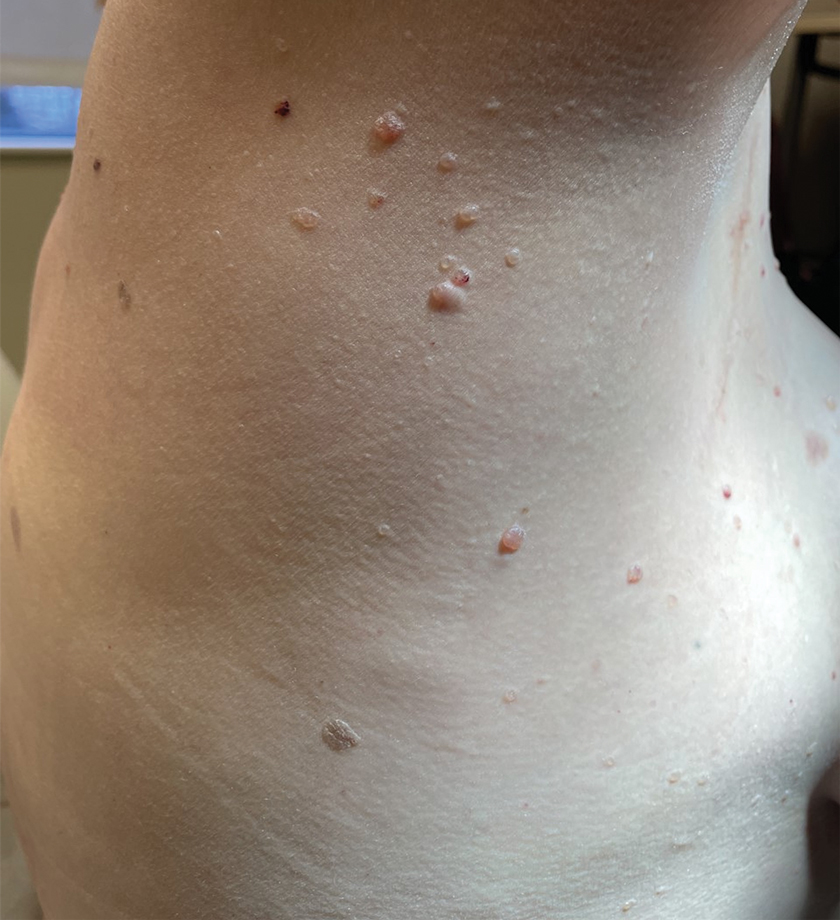
A 47-year-old woman with Cowden syndrome presented to the dermatology clinic with asymptomatic papules on and near the right breast that had increased in number over the last year. She had a medical history of breast cancer treated with mastectomy, chemotherapy, and radiation; papillary thyroid carcinoma treated with thyroidectomy and subsequent thyroid hormone replacement; dysplastic cerebellar gangliocytoma treated with surgical excision; and multiple cutaneous fibromas and angiolipomas. Physical examination revealed multiple clustered, 1- to 5-mm, translucent to red papules on the right breast, flank, and upper arm. A shave biopsy of a papule from the right lateral breast was performed.