User login
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
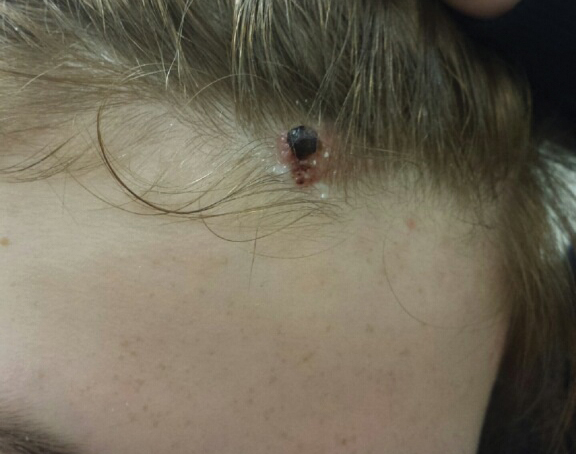
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
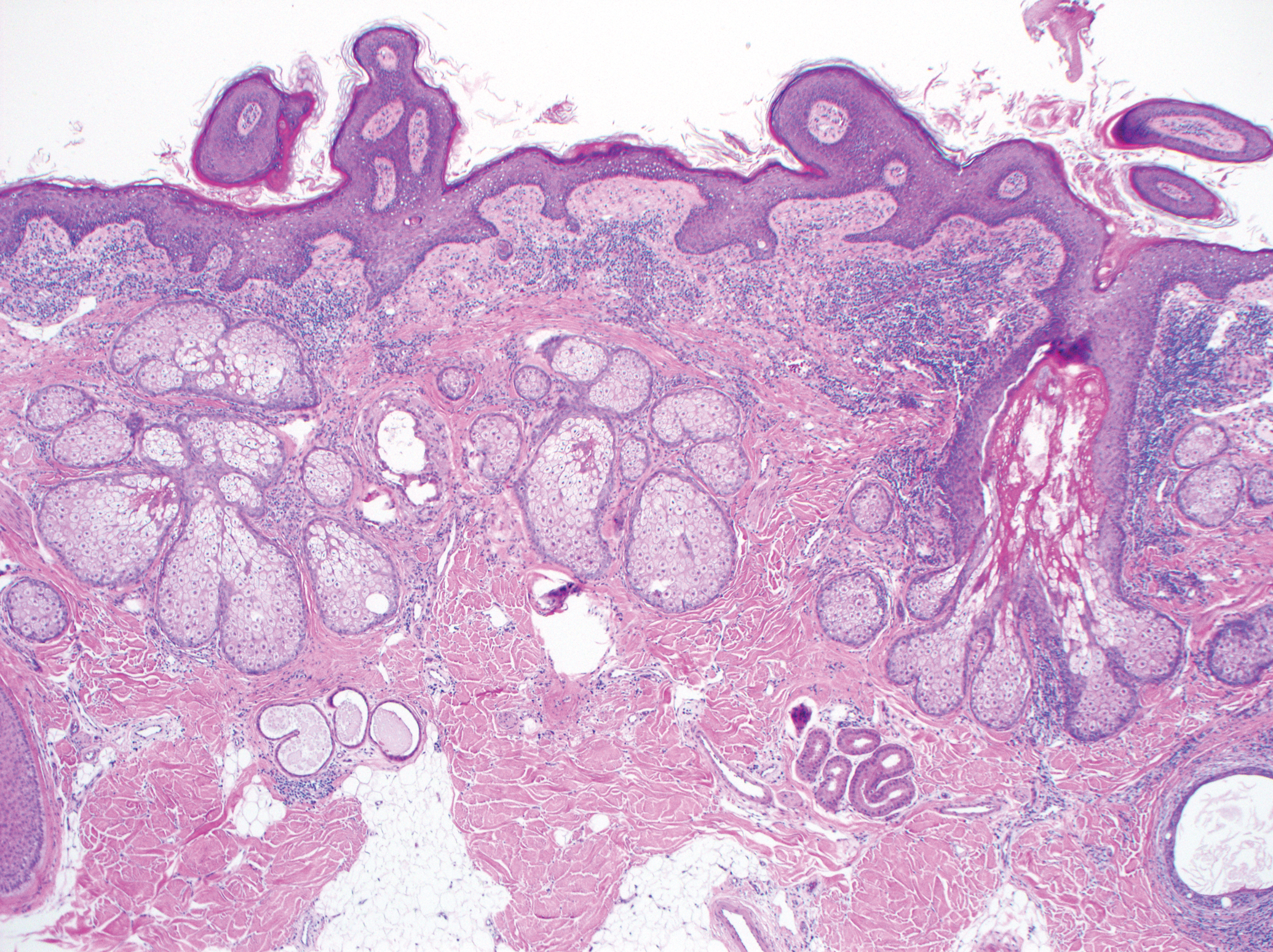
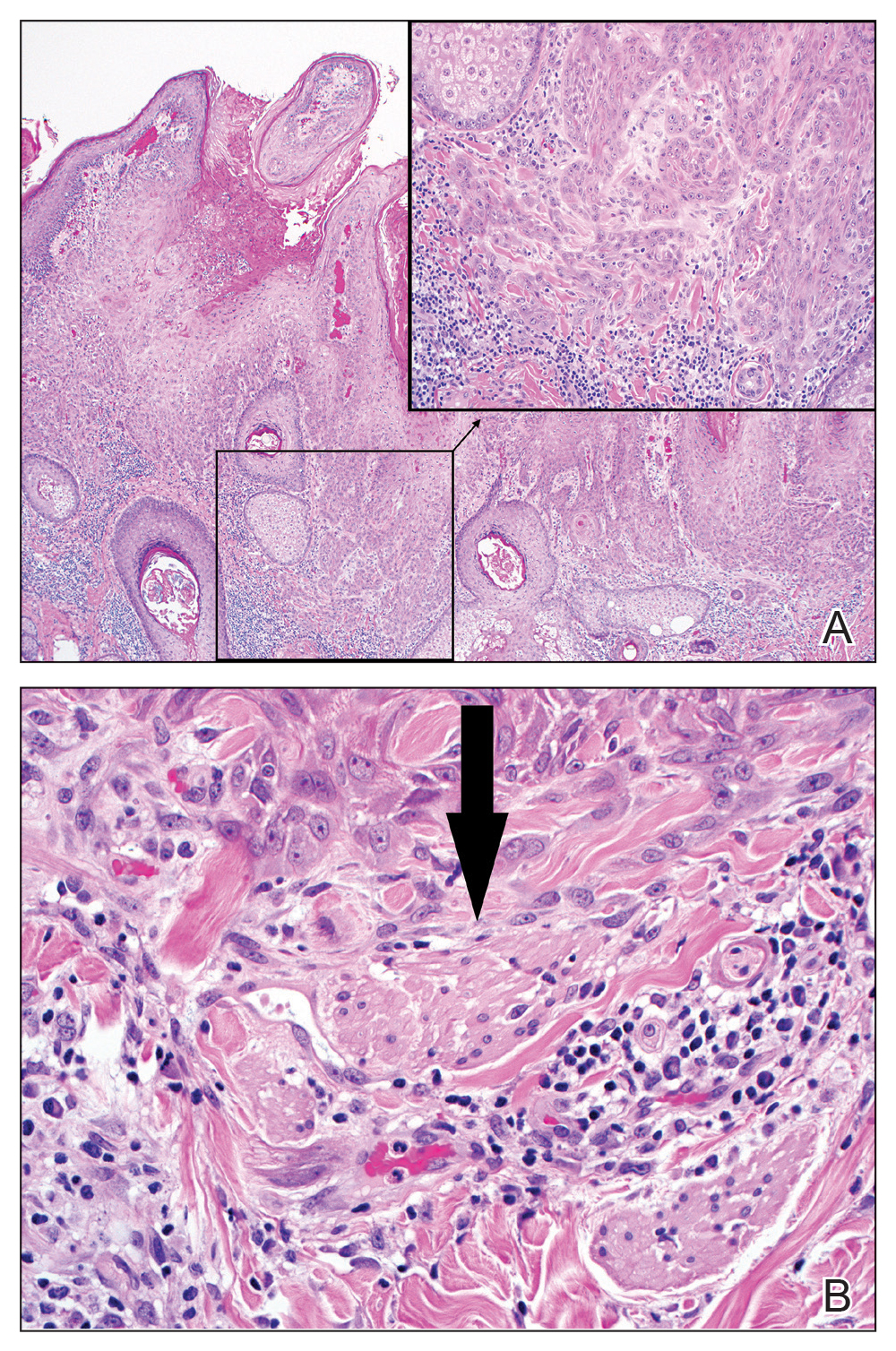
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
Practice Points
- Nevus sebaceus (NS) is frequently found on the scalp and may increase in size during puberty.
- Commonly found additional neoplasms within NS include trichoblastoma and syringocystadenoma papilliferum. Malignancies are possible but rare.