User login
To the Editor:
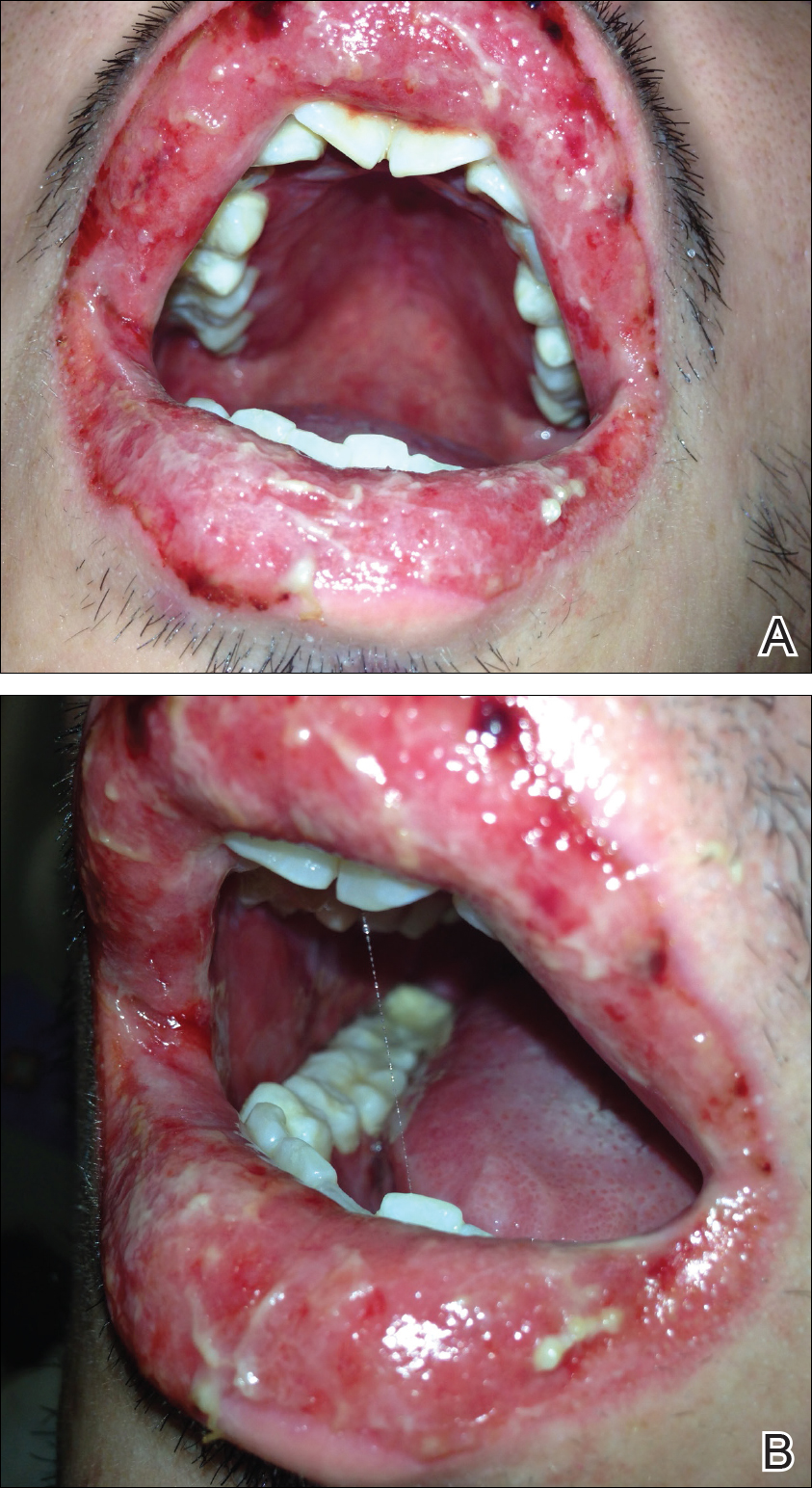
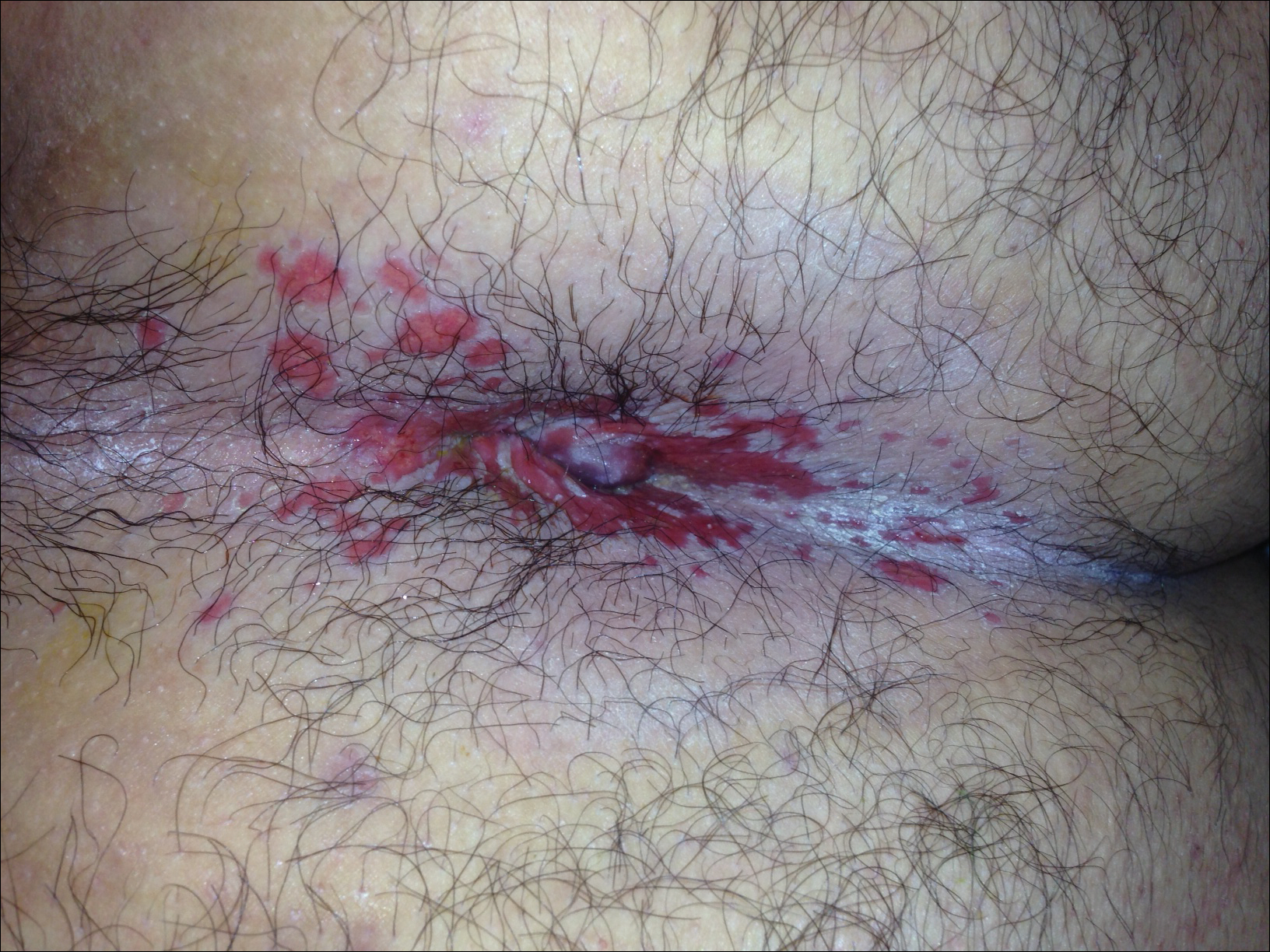
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.

One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
To the Editor:
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.



One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
To the Editor:
A 22-year-old obese man with untreated mild asthma diagnosed in childhood presented to the emergency department with cheilitis (Figure 1); conjunctivitis; and painful desquamation of the oral mucosa, penis (Figure 2), and perirectal area (Figure 3). Physical examination was notable for palpebral conjunctiva; mucosal involvement with stomatitis (Figure 1B); and isolated 0.5- to 2-cm erosions and ulcerations with positive Nikolsky sign of the scrotum (Figure 2), trunk, back, and arms and legs. Some areas had evidence of hemorrhagic crust, flaccid bullae, and denudation. Few scant targetoid lesions and dusky red macules on the trunk, face, palms, and soles also were present.



One week prior to presentation he had an episode of diarrhea and dyspnea with symptoms of mild heat stroke after working outdoors, and he self-treated with ibuprofen, which he had taken intermittently for years. He was subsequently seen at an outpatient clinic and was prescribed an albuterol inhaler for previously untreated childhood asthma. The patient stated that he inhaled 2 puffs every 6 hours for a total of 3 treatments. Shortly after the last dose, he noticed a tingling sensation of the oral mucosa that developed into a painful 2-cm bullous ulcer. Over the next 3 days, he developed several more oral ulcers and erosions. Three days before admission he developed dysuria and tense bullae at the glans penis. After admission, he developed cheilitis, conjunctivitis, dysuria, odynophagia, and dysphagia to solids. One day after admission, the patient had the onset of systemic symptoms, including cough with worsening dyspnea, fever, chills, hemoptysis, epistaxis, nausea, diarrhea, loss of appetite, joint pain, and myalgia. Review of systems was otherwise negative. A radiograph was performed at admission and was notable for mild atelectasis but was otherwise normal. The chest radiograph was negative for signs of perihilar lymphadenopathy, pleural effusion, pneumothorax, or lobar infiltrates suggestive of bacterial pneumonia. It also did not show signs of patchy opacities or air bronchograms suggestive of an interstitial pneumonia. On admission, he was started on acyclovir, fluconazole, methylprednisolone, nystatin, pantoprazole, acetaminophen, topical bacitracin, oxycodone, and topical silver nitrate.
At the time of admission our patient was afebrile with a normal heart rate, blood pressure, and respiratory rate. However, he was hypoxic, with a pulse oximetry of 86% on room air and 94% on 40% fraction of inspired oxygen. Complete blood cell count, electrolytes, and liver function tests were all within reference range. Urinalysis revealed evidence of scant red blood cells without pyuria, and the erythrocyte sedimentation rate and creatine kinase level were both elevated. Two blood cultures; sputum cultures; and polymerase chain reaction for Mycoplasma pneumoniae, herpes simplex virus, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus were negative. Human immunodeficiency virus panel, antinuclear antibody screen, and hepatitis B and C panels were all negative. Four punch biopsies were obtained showing full-thickness epidermal necrosis with neutrophils, few dyskeratotic cells, and sparse inflammatory infiltrate compatible with Stevens-Johnson syndrome (SJS).
After hospital admission, the patient’s mucosal desquamation progressively improved. By day 3, he required minimal supplemental oxygen with resolution of bowel symptoms and improved mucosal and skin findings. He was discharged on day 4 with supplemental oxygen and a 7-day course of prednisone, fluconazole, liquid oxycodone, pantoprazole, and acetaminophen. He showed continued improvement at a follow-up outpatient visit 2 days following discharge.
Stevens-Johnson syndrome is a rare severe drug reaction characterized by high fevers, mucosal erosions, tenderness, and skin detachment approximately 1 to 3 weeks after an inciting event.1,2 Although SJS has been linked to infections and less commonly to immunizations, in more than 80% of cases, SJS is strongly associated with a recent medication change.3 The classes of drugs that have been implicated in SJS most commonly include antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.4 Stevens-Johnson syndrome from drug reactions is not uncommon; however, SJS secondary to isolated albuterol use is rare.
Although it is presumed that albuterol was the key inciting factor in our patient’s case of SJS, it also is recognized that mucosal SJS can be associated with M pneumoniae infection. For this reason, we performed polymerase chain reaction for M pneumoniae as well as a chest radiograph to rule out this possibility. In addition, our patient had denied prolonged respiratory symptoms suggestive of a mycoplasma pneumonia infection, such as a prodrome of cough, myalgia, headache, sore throat, or fever. A report of 8 patients with documented SJS and M pneumoniae as well as a review of the literature also demonstrated a mean of 10 days of prodromal symptoms prior to the onset of mucosal lesions and/or a rash.5 However, mucosal SJS associated with mycoplasma pneumonia is an important clinical entity that should not be forgotten during the workup of a young patient with mucosal lesions or rash suggestive of SJS.
The exact etiology and mechanism of drug-induced SJS is not well understood at this time; however, evidence suggests that SJS is strongly linked to the host’s inability to detoxify drug metabolites.6,7 It has been postulated that SJS occurs secondary to a cell-mediated immune response, which activates cytotoxic T lymphocytes and subsequently results in keratinocyte apoptosis. Keratinocyte apoptosis occurs via the CD95-CD95 death receptor and soluble or membrane-bound ligand interaction.3,8,9 Stevens-Johnson syndrome is thought to occur from an interaction involving an HLA antigen–restricted presentation of drug metabolites to cytotoxic T cells, which can be further supported by evidence of strong genetic associations with HLA antigen alleles B15.02 and B58.01 in the cases of carbamazepine- and allopurinol-induced SJS, respectively.6,7 However, the genetic associations of specific HLA antigen alleles and polymorphisms with SJS and other cutaneous reactions is thought to be drug specific and HLA antigen subtype specific.7 Therefore, it is difficult to determine or correlate the clinical outcomes and manifestations of drug reactions in individualized patients. The precise mechanism of antigenicity of albuterol in initiating this cascade has not yet been determined. However, these investigations provide strong evidence for a correlation between specific HLA antigen haplotypes and occurrence of drug antigenicity resulting in SJS and other cutaneous reactions in susceptible patient populations.
Although the specific molecular pathway and etiology of SJS is not well delineated, pathology in combination with clinical correlation allows for diagnosis. Early-stage biopsies in SJS typically show apoptotic keratinocytes throughout the epidermis. Late-stage biopsies exhibit subepidermal blisters and full-thickness epidermal necrosis.1 Histopathology was performed on 4-mm punch biopsies of the chest and back and demonstrated full-thickness epidermal necrosis with neutrophils and a few dyskeratotic cells, likely representing a late stage of epidermal involvement. Given the predominance of neutrophils, other diagnostic considerations based solely on the biopsy results included contact dermatitis or phototoxic dermatitis. The remaining inflammatory infiltrate was sparse. Immunofluorescence was pan-negative.
This report illustrates a rare case of SJS from isolated albuterol use. This adverse drug reaction has not been well reported in the literature and may be an important consideration in the management of a patient with asthma.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
- Stern RS. Clinical practice. exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
- Tartarone A, Lerose R. Stevens-Johnson syndrome and toxic epidermal necrolysis: what do we know? Ther Drug Monit. 2010;32:669-672.
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98:998-1003.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. the EuroSCAR-study. J Invest Dermatol. 2008;128:35-44.
- Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30:42-49.
- Chung WH, Hung SI. Genetic markers and danger signals in Stevens-Johnson syndrome and toxic epidermal necrolysis [published online October 25, 2010]. Allergol Int. 2010;59:325-332.
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196.
- Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401-431.
- Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822-832.
Practice Points
- Think of Stevens-Johnson syndrome when new skin lesions are seen after any new medication is started.
- Perform a full-body examination to assess the extent of skin eruptions.
- When a medication is atypical for skin eruption, it becomes necessary to assess for other systemic causes and confirm pathologic results on skin biopsy.
