User login
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
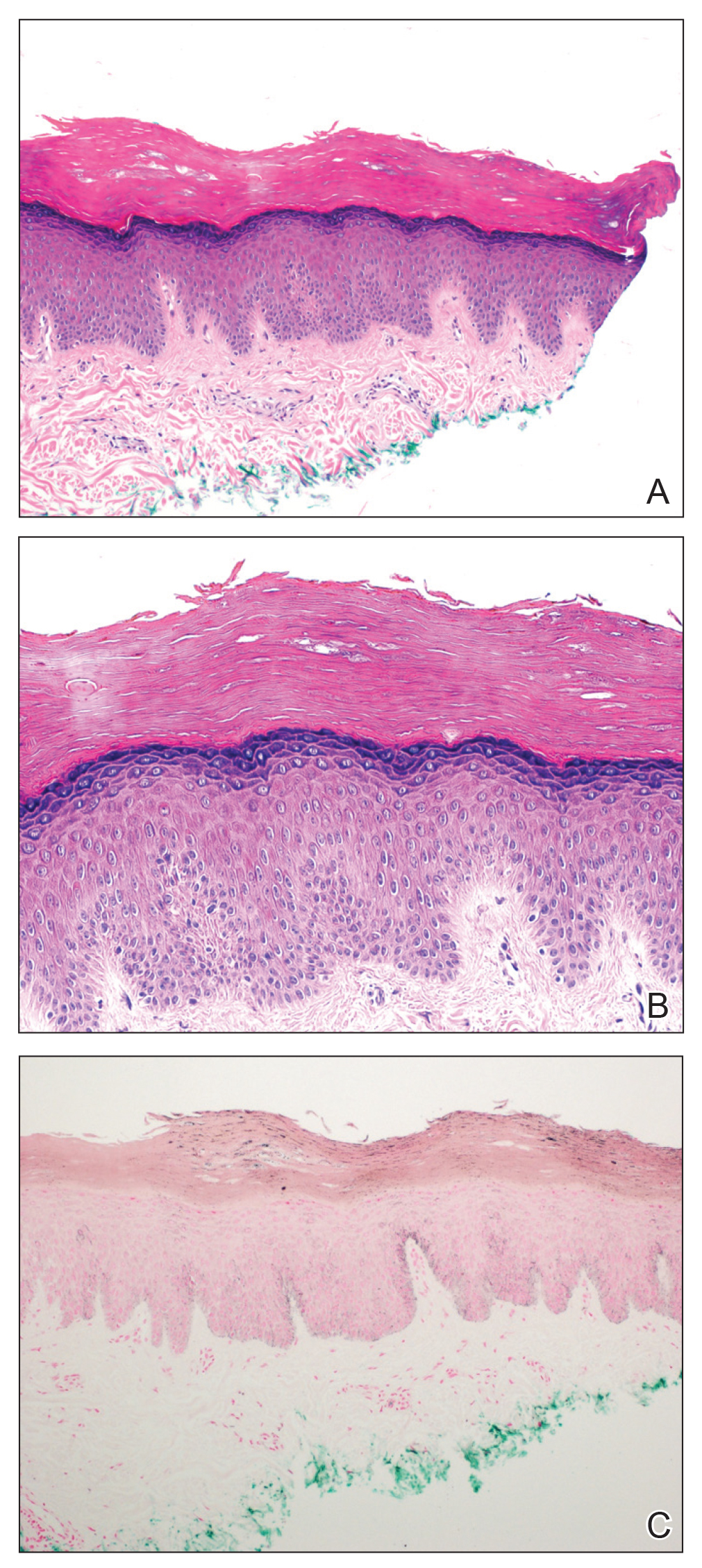
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
Practice Points
- Patient education on the benefits and risks associated with sunless tanners is critical when using these products.
- Sunless tanners containing dihydroxyacetone potentially can lead to persistent hyperpigmented patches on areas of contact.
- Skin biopsy showing hyperpigmented parakeratosis along with pigmentation of the stratum corneum can aid in diagnosis.