User login
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.
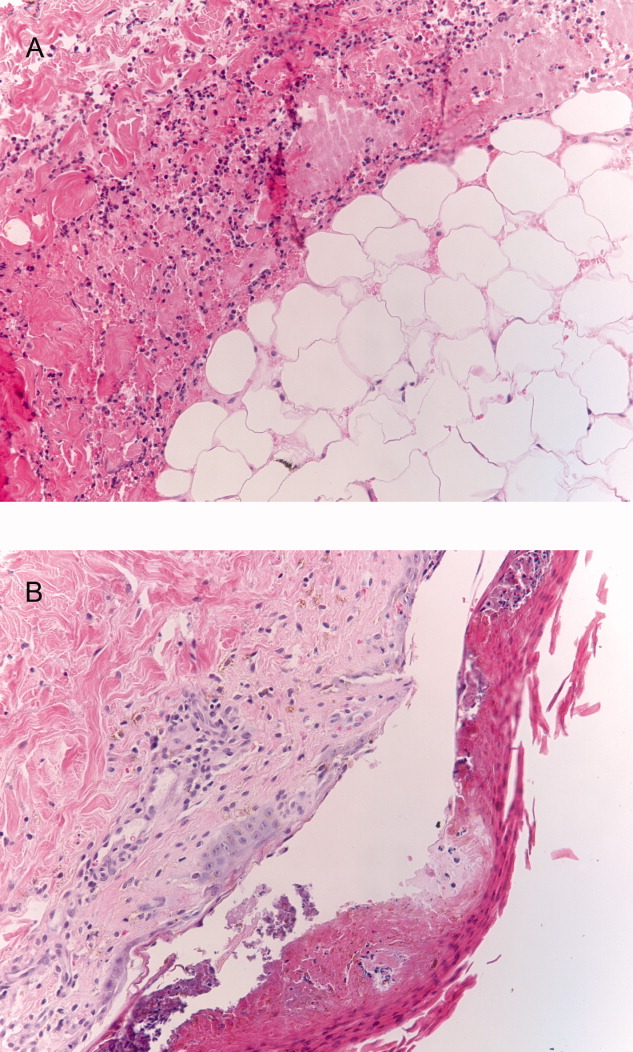
Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.