User login
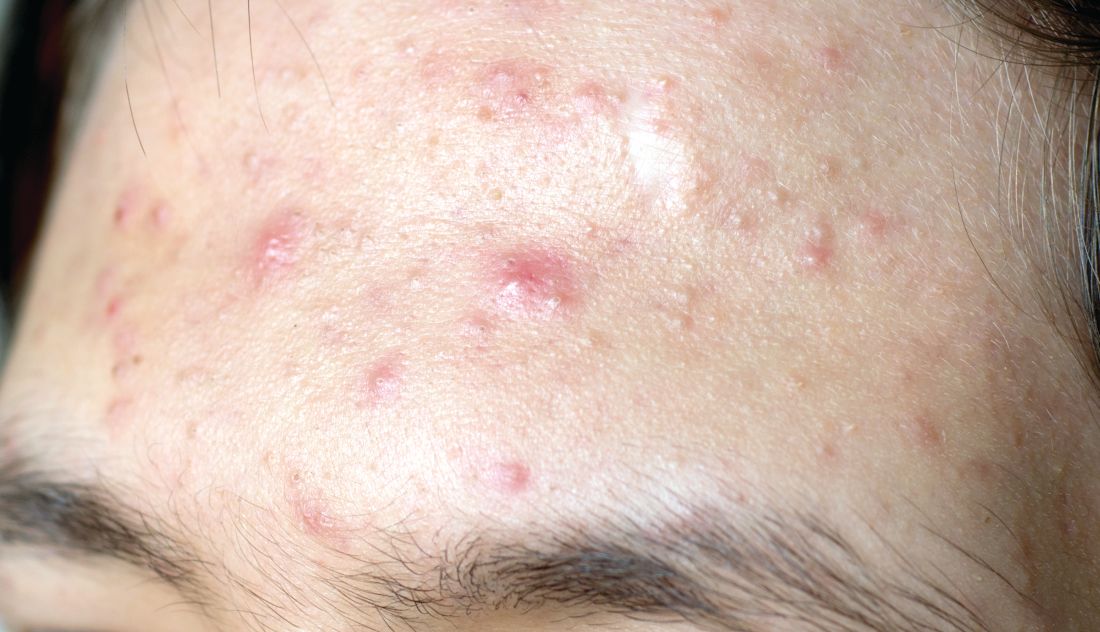
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
AT MOAS 2022