User login
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
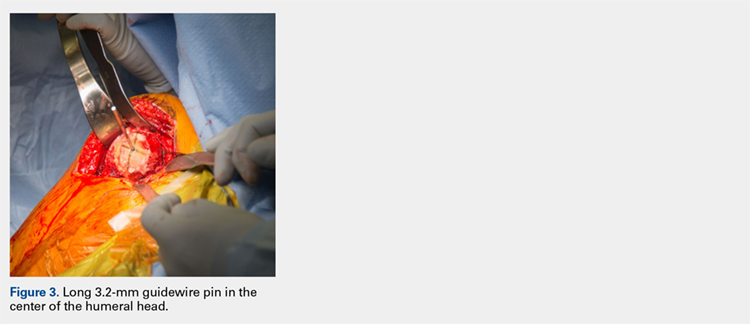
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).
Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

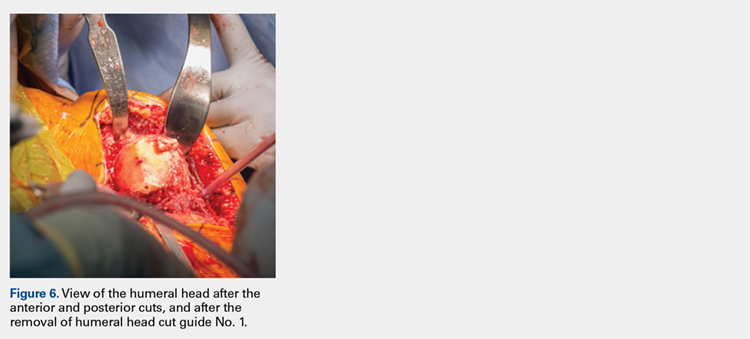
The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).
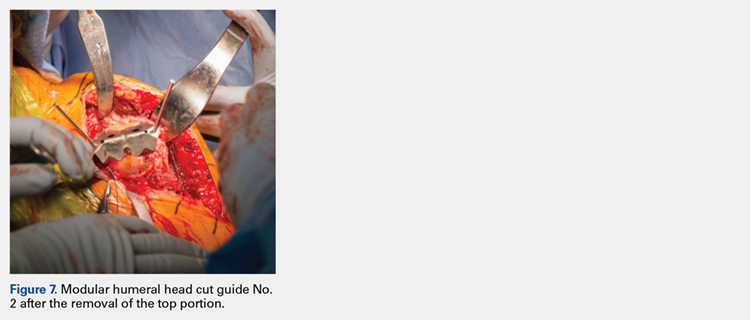
The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).
The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
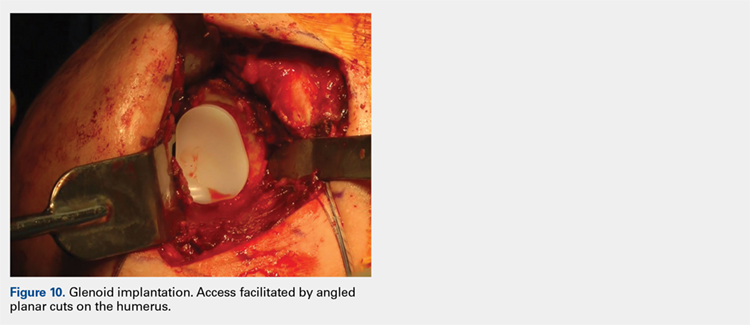
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

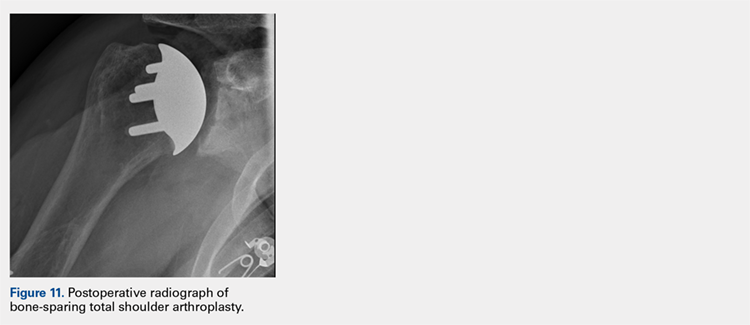
After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.
DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12
Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
TAKE-HOME POINTS
- Bone-preserving shoulder arthroplasty is now available and rapidly growing in the US.
- The calibrated, multiplanar instruments and prosthesis shown here allow surgeons to recreate the normal humerus shape with high precision.
- The elliptical, non-spherical design of the humerus prosthesis has shown improved shoulder kinematics compared to standard spherical prostheses.
- The implant rests on dense bone proximal to the anatomic neck where bone support is strong.
- Glenoid implant insertion is routinely performed using this technique and access is facilitated by the angled bone resections.