User login
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio
Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
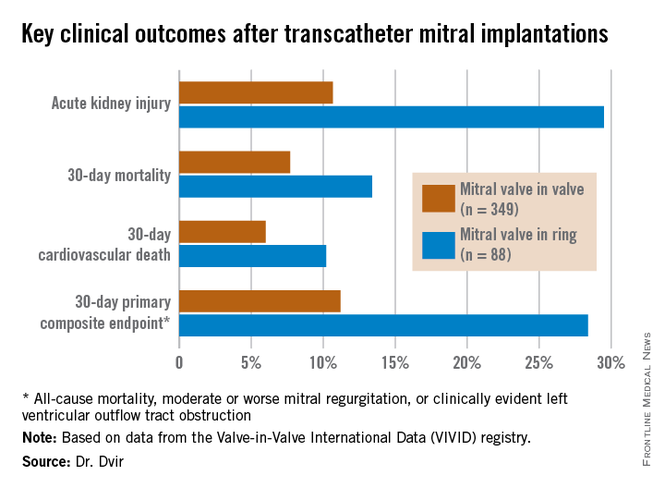
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio

Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio

Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
AT EuroPCR 2015
Key clinical point: Transcatheter mitral valve-in-valve procedures for very high surgical risk patients with a failed bioprosthetic valve yield far superior outcomes, compared with mitral valve-in-ring procedures.
Major finding: The 30-day composite adverse outcome rate comprised of death, moderate or severe mitral regurgitation, or clinically evident left ventricular outflow tract obstruction occurred in 11.2% of patients who underwent a transcatheter mitral valve-in-valve procedure, compared with 28.4% of those with a transcatheter valve-in-ring procedure.
Data source: The ongoing VIVID registry includes patients on six continents undergoing transcatheter implantation of aortic, mitral, and/or tricuspid valves after failure of an earlier bioprosthetic valve.
Disclosures: The presenter reported having no relevant financial conflicts.

