User login
EuroPCR 2015
Transcatheter mitral valve-in-valve implantation advantageous in failed bioprosthetic valves
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio
Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
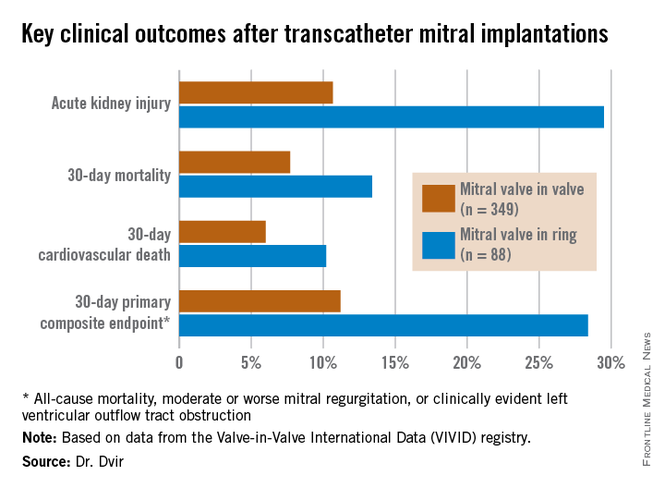
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio

Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
PARIS – Transcatheter mitral valve-in-valve procedures in patients with a failed bioprosthetic mitral valve provide superior clinical results, compared with valve-in-ring implantations, according to the latest data from the VIVID registry.
“Obviously, these results have numerous implications for the interventional community: for surgeons, who employ bioprostheses and rings, and for the cardiovascular industry that designs transcatheter strategies for mitral valve and ring implantations,” Dr. Danny Dvir said in presenting the VIVID update at the annual congress of the European Association of Percutaneous Cardiovascular Interventio

Bioprosthetic rather than mechanical valves are increasingly popular in open-heart valve replacement operations because they avoid the need for lifelong oral anticoagulation. But over time bioprosthetic valves often fail. And that event creates a need for a high-risk, repeat open surgery or a far less invasive transcatheter valve replacement.
Lots of data are available regarding the effectiveness and limitations of transcatheter aortic valve-in-valve procedures in patients with failed bioprosthetic aortic valves, including a previous report from the VIVID (Valve-in-Valve International Data registry) by Dr. Dvir and coworkers (JAMA 2014;312:162-70). However, very little data exist about the effectiveness of mitral valve-in-valve and valve-in-ring procedures, noted Dr. Dvir of St. Paul’s Hospital and the University of British Columbia, Vancouver.
The VIVID registry is drawn from 94 sites on six continents. Dr. Dvir presented an analysis of 437 transcatheter mitral implantations in patients with failed bioprosthetic valves. The study population consisted of 349 patients with mitral valve-in-valve (VIV) and 88 with valve-in-ring (VIR) procedures.
The average Society of Thoracic Surgeons (STS) score was 12.9. “This is one of the highest-risk groups of patients that you’ll ever see,” the cardiologist commented.
Mitral regurgitation was the mechanism of bioprosthetic valve failure in 45% of cases, stenosis in 23%, and a combination of the two in the remainder. The median time from placement of the initial bioprosthetic valve to the transcatheter VIV or VIR procedure was 9 years.
Transcatheter access for the mitral VIV or VIR was transapical in 79% of cases, directly through the left atrium in 2.5%, and by the increasingly popular transseptal route in the remainder. The median follow-up post procedure was 408 days.
Mitral VIV procedures looked significantly better from the standpoint of procedural characteristics. Moderate or severe mitral regurgitation was present at the conclusion of the procedure in 2.6% of patients undergoing mitral VIV, compared with 14.8% who had a VIR procedure.
“We were a bit shocked to see that difference between the two groups,” Dr. Dvir said.
Implantation of a small surgical valve of 25 mm or less was the major independent predictor of having an elevated gradient post procedure, with an adjusted odds ratio of 3.7.
In addition, the mean valve area was 1.99 cm2 in the VIV group vs. 2.33 cm2 in the VIR group. Postinflation was employed in 3.2% of the VIV group, compared with 22.7% of the VIR group. Left ventricular outflow tract obstruction – a potentially fatal complication – occurred in 2.6% of the mitral VIV group and was significantly more common at 8% in the VIR group.
Mitral VIR procedures were associated with markedly worse clinical outcomes. Highlighting the 11.2% rate of the 30-day primary composite endpoint in the mitral VIV group, Dr. Dvir commented, “I must say, I think that is a very reasonable number given that we’re talking about a very high–risk group of patients. For this group of patients having a very high STS score, reaching good results in 30 days in the mitral valve-in-valve group is a good signal for the regulatory groups and for the community that this is a good procedure.”
In contrast, the 28.4% rate of the composite endpoint in the VIR group “is a big disappointment; almost one-third of patients undergoing mitral valve in ring experience the composite adverse event endpoint at 30 days. There is an issue there,” he added.
Dr. Dvir reported having no relevant financial conflicts.
AT EuroPCR 2015
Key clinical point: Transcatheter mitral valve-in-valve procedures for very high surgical risk patients with a failed bioprosthetic valve yield far superior outcomes, compared with mitral valve-in-ring procedures.
Major finding: The 30-day composite adverse outcome rate comprised of death, moderate or severe mitral regurgitation, or clinically evident left ventricular outflow tract obstruction occurred in 11.2% of patients who underwent a transcatheter mitral valve-in-valve procedure, compared with 28.4% of those with a transcatheter valve-in-ring procedure.
Data source: The ongoing VIVID registry includes patients on six continents undergoing transcatheter implantation of aortic, mitral, and/or tricuspid valves after failure of an earlier bioprosthetic valve.
Disclosures: The presenter reported having no relevant financial conflicts.
EUROPCR: New technology shows early promise for transcatheter tricuspid valve repair
PARIS – Early highly preliminary results from the first-in-human study of percutaneous repair of a failing tricuspid valve indicate that the transcatheter procedure appears safe, durable, and effective – at least through the first 6 months.
“Even though the one-grade reduction of tricuspid regurgitation on color Doppler flow imaging is modest, the impact in terms of clinical improvement seems very encouraging and warrants pursuing this innovative technique,” Dr. Jean-Michel Juliard said in presenting the early findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The three-country, prospective, single-arm study is aimed at winning European regulatory approval for 4tech Cardio’s TriCinch System. The study includes 24 patients with severe functional tricuspid regurgitation. Dr. Juliard, a cardiologist at Bichat Hospital in Paris, presented 6-month follow-up data on two patients and 3-month follow-up for a third.
First, a bit about the procedure. It’s conducted under general anesthesia and relies upon transesophageal and intracardiac echocardiographic guidance. The objectives are to reduce the effective cross-sectional valve area by diminishing the septo-lateral dimension of the tricuspid annulus diameter, restore tricuspid valve leaflet coaptation, and relieve symptoms.
The 4tech delivery system consists of two parts. To begin, a guidewire is placed in the right coronary artery, a 24-French sheath is introduced via the right femoral vein, and an exchange guidewire is positioned in the right ventricular apex. The first part of the delivery system is then inserted in order to place an anchor on the tricuspid valve annulus in the vicinity of the antero-posterior commissure.
“This is probably the most difficult part of the whole procedure,” according to Dr. Juliard. “It is done using intracoronary and transesophageal echo guidance.”
Once the anchor is well positioned, the second part of the system is advanced, locked into the first part, and tension is applied under echocardiographic control in order to implant a nitinol self-expanding stent in the inferior vena cava. As soon as traction is applied, the tricuspid regurgitation decreases. Once the correction process is completed, the TriCinch delivery system and venous introducers are removed. For study purposes, dosing of diuretics remained unchanged post procedure.
In the first 6 months, the tricuspid septo-lateral distance improved from 52 to 42 mm in one patient and from 45 to 40 mm in another. A third patient had improvement from 43 to 34 mm at 3 months of follow-up.
In terms of clinical improvement, two patients went from New York Heart Association functional class III to class II, and another went from class III to class I. Six-minute walk distance increased from a baseline of 320 m to 367 m at 6 months of follow-up in one patient and from 400 m to 750 m in another, with a 3-month improvement from 160 to 280 m in the third patient.
Serial imaging studies showed device stability over time, with no migration and no stent thrombosis.
In response to an audience question, Dr. Juliard conceded that at this early stage in the development of the procedure, the placebo effect can’t be ruled out as a possible explanation for the observed strong clinical improvement despite what he conceded was “very modest” reduction in tricuspid regurgitation. Time will tell.
“I hope it is not a placebo effect, but as we maintain the same dose of diuretics, it does seem that the patients feel better,” he added.
The rationale for developing a transcatheter solution to functional tricuspid regurgitation, especially one that’s compatible with concomitant transcatheter mitral valve procedures, as the TriCinch System is intended to be, lies in the wealth of evidence that tricuspid regurgitation is associated with increased mortality, substantial morbidity, and severely impaired quality of life due to the need for repeated hospitalizations. Surgery for patients with tricuspid regurgitation is often extremely high risk because affected patients typically have right ventricular dysfunction, pulmonary hypertension, and/or previous valve surgery.
The ongoing study is funded by 4tech Cardio. Dr. Juliard reported having no financial conflicts.
PARIS – Early highly preliminary results from the first-in-human study of percutaneous repair of a failing tricuspid valve indicate that the transcatheter procedure appears safe, durable, and effective – at least through the first 6 months.
“Even though the one-grade reduction of tricuspid regurgitation on color Doppler flow imaging is modest, the impact in terms of clinical improvement seems very encouraging and warrants pursuing this innovative technique,” Dr. Jean-Michel Juliard said in presenting the early findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The three-country, prospective, single-arm study is aimed at winning European regulatory approval for 4tech Cardio’s TriCinch System. The study includes 24 patients with severe functional tricuspid regurgitation. Dr. Juliard, a cardiologist at Bichat Hospital in Paris, presented 6-month follow-up data on two patients and 3-month follow-up for a third.
First, a bit about the procedure. It’s conducted under general anesthesia and relies upon transesophageal and intracardiac echocardiographic guidance. The objectives are to reduce the effective cross-sectional valve area by diminishing the septo-lateral dimension of the tricuspid annulus diameter, restore tricuspid valve leaflet coaptation, and relieve symptoms.
The 4tech delivery system consists of two parts. To begin, a guidewire is placed in the right coronary artery, a 24-French sheath is introduced via the right femoral vein, and an exchange guidewire is positioned in the right ventricular apex. The first part of the delivery system is then inserted in order to place an anchor on the tricuspid valve annulus in the vicinity of the antero-posterior commissure.
“This is probably the most difficult part of the whole procedure,” according to Dr. Juliard. “It is done using intracoronary and transesophageal echo guidance.”
Once the anchor is well positioned, the second part of the system is advanced, locked into the first part, and tension is applied under echocardiographic control in order to implant a nitinol self-expanding stent in the inferior vena cava. As soon as traction is applied, the tricuspid regurgitation decreases. Once the correction process is completed, the TriCinch delivery system and venous introducers are removed. For study purposes, dosing of diuretics remained unchanged post procedure.
In the first 6 months, the tricuspid septo-lateral distance improved from 52 to 42 mm in one patient and from 45 to 40 mm in another. A third patient had improvement from 43 to 34 mm at 3 months of follow-up.
In terms of clinical improvement, two patients went from New York Heart Association functional class III to class II, and another went from class III to class I. Six-minute walk distance increased from a baseline of 320 m to 367 m at 6 months of follow-up in one patient and from 400 m to 750 m in another, with a 3-month improvement from 160 to 280 m in the third patient.
Serial imaging studies showed device stability over time, with no migration and no stent thrombosis.
In response to an audience question, Dr. Juliard conceded that at this early stage in the development of the procedure, the placebo effect can’t be ruled out as a possible explanation for the observed strong clinical improvement despite what he conceded was “very modest” reduction in tricuspid regurgitation. Time will tell.
“I hope it is not a placebo effect, but as we maintain the same dose of diuretics, it does seem that the patients feel better,” he added.
The rationale for developing a transcatheter solution to functional tricuspid regurgitation, especially one that’s compatible with concomitant transcatheter mitral valve procedures, as the TriCinch System is intended to be, lies in the wealth of evidence that tricuspid regurgitation is associated with increased mortality, substantial morbidity, and severely impaired quality of life due to the need for repeated hospitalizations. Surgery for patients with tricuspid regurgitation is often extremely high risk because affected patients typically have right ventricular dysfunction, pulmonary hypertension, and/or previous valve surgery.
The ongoing study is funded by 4tech Cardio. Dr. Juliard reported having no financial conflicts.
PARIS – Early highly preliminary results from the first-in-human study of percutaneous repair of a failing tricuspid valve indicate that the transcatheter procedure appears safe, durable, and effective – at least through the first 6 months.
“Even though the one-grade reduction of tricuspid regurgitation on color Doppler flow imaging is modest, the impact in terms of clinical improvement seems very encouraging and warrants pursuing this innovative technique,” Dr. Jean-Michel Juliard said in presenting the early findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The three-country, prospective, single-arm study is aimed at winning European regulatory approval for 4tech Cardio’s TriCinch System. The study includes 24 patients with severe functional tricuspid regurgitation. Dr. Juliard, a cardiologist at Bichat Hospital in Paris, presented 6-month follow-up data on two patients and 3-month follow-up for a third.
First, a bit about the procedure. It’s conducted under general anesthesia and relies upon transesophageal and intracardiac echocardiographic guidance. The objectives are to reduce the effective cross-sectional valve area by diminishing the septo-lateral dimension of the tricuspid annulus diameter, restore tricuspid valve leaflet coaptation, and relieve symptoms.
The 4tech delivery system consists of two parts. To begin, a guidewire is placed in the right coronary artery, a 24-French sheath is introduced via the right femoral vein, and an exchange guidewire is positioned in the right ventricular apex. The first part of the delivery system is then inserted in order to place an anchor on the tricuspid valve annulus in the vicinity of the antero-posterior commissure.
“This is probably the most difficult part of the whole procedure,” according to Dr. Juliard. “It is done using intracoronary and transesophageal echo guidance.”
Once the anchor is well positioned, the second part of the system is advanced, locked into the first part, and tension is applied under echocardiographic control in order to implant a nitinol self-expanding stent in the inferior vena cava. As soon as traction is applied, the tricuspid regurgitation decreases. Once the correction process is completed, the TriCinch delivery system and venous introducers are removed. For study purposes, dosing of diuretics remained unchanged post procedure.
In the first 6 months, the tricuspid septo-lateral distance improved from 52 to 42 mm in one patient and from 45 to 40 mm in another. A third patient had improvement from 43 to 34 mm at 3 months of follow-up.
In terms of clinical improvement, two patients went from New York Heart Association functional class III to class II, and another went from class III to class I. Six-minute walk distance increased from a baseline of 320 m to 367 m at 6 months of follow-up in one patient and from 400 m to 750 m in another, with a 3-month improvement from 160 to 280 m in the third patient.
Serial imaging studies showed device stability over time, with no migration and no stent thrombosis.
In response to an audience question, Dr. Juliard conceded that at this early stage in the development of the procedure, the placebo effect can’t be ruled out as a possible explanation for the observed strong clinical improvement despite what he conceded was “very modest” reduction in tricuspid regurgitation. Time will tell.
“I hope it is not a placebo effect, but as we maintain the same dose of diuretics, it does seem that the patients feel better,” he added.
The rationale for developing a transcatheter solution to functional tricuspid regurgitation, especially one that’s compatible with concomitant transcatheter mitral valve procedures, as the TriCinch System is intended to be, lies in the wealth of evidence that tricuspid regurgitation is associated with increased mortality, substantial morbidity, and severely impaired quality of life due to the need for repeated hospitalizations. Surgery for patients with tricuspid regurgitation is often extremely high risk because affected patients typically have right ventricular dysfunction, pulmonary hypertension, and/or previous valve surgery.
The ongoing study is funded by 4tech Cardio. Dr. Juliard reported having no financial conflicts.
AT EUROPCR 2015
Key clinical point: A novel technology for transcatheter repair of a failing tricuspid valve shows early promise of safety and efficacy.
Major finding: At 3-6 months of follow-up, the first three participants in a first-in-human study of percutaneous tricuspid valve repair show substantial clinical benefits.
Data source: This is a prospective, three-center, nonrandomized, single-arm study of a novel percutaneous therapy in 24 patients with severe functional tricuspid regurgitation.
Disclosures: This ongoing study is funded by 4tech Cardio. The presenter reported having no financial conflicts.
ZEUS: Second-generation DES with 30 days DAPT best in bleeding-risk patients
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
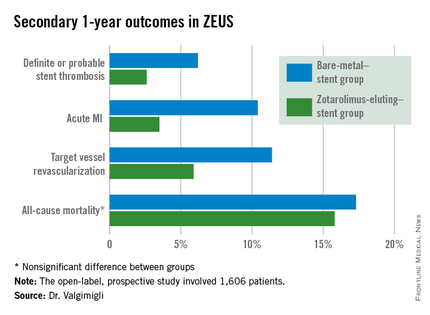
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.
Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
AT EUROPCR 2015
Key clinical point: High bleeding risk patients fare significantly better with a second-generation drug-eluting stent and 30 days of dual antiplatelet therapy than with a bare-metal stent.
Major finding: The 1-year incidence of major adverse cardiovascular events was 29.6% in high bleeding risk patients who received a bare-metal stent and 22.6% in those who got a second-generation zotarolimus-eluting stent with 30 days of dual antiplatelet therapy.
Data source: This was a prespecified analysis of 828 high bleeding risk patients randomized to a bare-metal stent or a second-generation zotarolimus-eluting stent in conjunction with 30 days of DAPT and then followed prospectively for 1 year.
Disclosures: The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. The presenter serves as a consultant and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
Bivalirudin in STEMI has low real-world stent thrombosis rate
PARIS – Antithrombotic therapy with bivalirudin for primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction may have been unfairly tarnished as having a high stent thrombosis rate, according to a large, prospective, observational cohort study.
A new analysis from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) showed similarly low stent thrombosis rates within 30 days following primary PCI for STEMI regardless of whether the antithrombotic regimen involved bivalirudin (Angiomax), heparin only, or a glycoprotein IIb/IIIa inhibitor, Dr. Per Grimfjard reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The SCAAR analysis captured all STEMI patients undergoing primary PCI in Sweden from 2007 through mid-2014. These data reflect real-world interventional practice in Sweden and elsewhere, where bivalirudin is typically administered in a prolonged infusion to protect against early stent thrombosis. In contrast, the randomized trials that linked bivalirudin to high stent thrombosis rates featured protocols in which the drug was stopped immediately after the procedure, noted Dr. Grimfjard, an interventional cardiologist at Uppsala (Sweden) University.
“These are nationwide Swedish numbers, and they are complete. We think the numbers are reassuring in that respect,” he said.
Session chair Dr. Andreas Baumbach said the Swedish data are consistent with his own experience in using bivalirudin in primary PCI for STEMI.
“The headline last year was that bivalirudin has a high stent thrombosis rate. It made the newspapers everywhere. But we never saw that, and we always thought that the difference might be in how we used the drug. There’s a new headline now, that this high stent thrombosis rate is not seen in clinical practice. The practice differs from the randomized trials, and the outcomes differ as well,” observed Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
In SCAAR, the 30-day rate of definite, angiographically proven stent thrombosis was 0.84% in 16,860 bivalirudin-treated patients, 0.94% in 3,182 who got heparin only, and 0.83% in 11,216 glycoprotein IIb/IIIa inhibitor recipients. These numeric differences weren’t statistically significant.
All-cause mortality 1 year post-PCI was 9.1% in patients with no stent thrombosis, 16.1% in those who experienced stent thrombosis within 1 day post PCI, and 23.0% in those whose stent thrombosis occurred on days 2-30. Dr. Grimfjard speculated that the explanation for the numerically higher 1-year all-cause mortality rate in patients whose stent thrombosis occurred on days 2-30 as opposed to day 0-1 is probably that they were more likely to have left the hospital when stent thrombosis occurred. That would translate to a longer time to repeat revascularization, hence a larger MI, more heart failure and arrhythmia, and thus a higher long-term risk of death.
Several audience members commented that they weren’t sure what to make of the observational Swedish data because of the looming presence of several potential confounders. For one, clinical practice trends changed considerably during the 7-year time frame of the study, as evidenced by the fact that the use of drug-eluting stents was far more common in bivalirudin-treated patients than in the glycoprotein IIb/IIIa inhibitor group. Also, Swedish cardiologists who put their STEMI patients on bivalirudin were more likely to utilize the more modern radial artery access in performing primary PCI; their practice may have differed from their colleagues’ in other, unrecorded ways as well, it was noted.
Dr. Grimfjard reported having no financial conflicts regarding the study, which was conducted free of commercial support.
PARIS – Antithrombotic therapy with bivalirudin for primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction may have been unfairly tarnished as having a high stent thrombosis rate, according to a large, prospective, observational cohort study.
A new analysis from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) showed similarly low stent thrombosis rates within 30 days following primary PCI for STEMI regardless of whether the antithrombotic regimen involved bivalirudin (Angiomax), heparin only, or a glycoprotein IIb/IIIa inhibitor, Dr. Per Grimfjard reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The SCAAR analysis captured all STEMI patients undergoing primary PCI in Sweden from 2007 through mid-2014. These data reflect real-world interventional practice in Sweden and elsewhere, where bivalirudin is typically administered in a prolonged infusion to protect against early stent thrombosis. In contrast, the randomized trials that linked bivalirudin to high stent thrombosis rates featured protocols in which the drug was stopped immediately after the procedure, noted Dr. Grimfjard, an interventional cardiologist at Uppsala (Sweden) University.
“These are nationwide Swedish numbers, and they are complete. We think the numbers are reassuring in that respect,” he said.
Session chair Dr. Andreas Baumbach said the Swedish data are consistent with his own experience in using bivalirudin in primary PCI for STEMI.
“The headline last year was that bivalirudin has a high stent thrombosis rate. It made the newspapers everywhere. But we never saw that, and we always thought that the difference might be in how we used the drug. There’s a new headline now, that this high stent thrombosis rate is not seen in clinical practice. The practice differs from the randomized trials, and the outcomes differ as well,” observed Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
In SCAAR, the 30-day rate of definite, angiographically proven stent thrombosis was 0.84% in 16,860 bivalirudin-treated patients, 0.94% in 3,182 who got heparin only, and 0.83% in 11,216 glycoprotein IIb/IIIa inhibitor recipients. These numeric differences weren’t statistically significant.
All-cause mortality 1 year post-PCI was 9.1% in patients with no stent thrombosis, 16.1% in those who experienced stent thrombosis within 1 day post PCI, and 23.0% in those whose stent thrombosis occurred on days 2-30. Dr. Grimfjard speculated that the explanation for the numerically higher 1-year all-cause mortality rate in patients whose stent thrombosis occurred on days 2-30 as opposed to day 0-1 is probably that they were more likely to have left the hospital when stent thrombosis occurred. That would translate to a longer time to repeat revascularization, hence a larger MI, more heart failure and arrhythmia, and thus a higher long-term risk of death.
Several audience members commented that they weren’t sure what to make of the observational Swedish data because of the looming presence of several potential confounders. For one, clinical practice trends changed considerably during the 7-year time frame of the study, as evidenced by the fact that the use of drug-eluting stents was far more common in bivalirudin-treated patients than in the glycoprotein IIb/IIIa inhibitor group. Also, Swedish cardiologists who put their STEMI patients on bivalirudin were more likely to utilize the more modern radial artery access in performing primary PCI; their practice may have differed from their colleagues’ in other, unrecorded ways as well, it was noted.
Dr. Grimfjard reported having no financial conflicts regarding the study, which was conducted free of commercial support.
PARIS – Antithrombotic therapy with bivalirudin for primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction may have been unfairly tarnished as having a high stent thrombosis rate, according to a large, prospective, observational cohort study.
A new analysis from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) showed similarly low stent thrombosis rates within 30 days following primary PCI for STEMI regardless of whether the antithrombotic regimen involved bivalirudin (Angiomax), heparin only, or a glycoprotein IIb/IIIa inhibitor, Dr. Per Grimfjard reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The SCAAR analysis captured all STEMI patients undergoing primary PCI in Sweden from 2007 through mid-2014. These data reflect real-world interventional practice in Sweden and elsewhere, where bivalirudin is typically administered in a prolonged infusion to protect against early stent thrombosis. In contrast, the randomized trials that linked bivalirudin to high stent thrombosis rates featured protocols in which the drug was stopped immediately after the procedure, noted Dr. Grimfjard, an interventional cardiologist at Uppsala (Sweden) University.
“These are nationwide Swedish numbers, and they are complete. We think the numbers are reassuring in that respect,” he said.
Session chair Dr. Andreas Baumbach said the Swedish data are consistent with his own experience in using bivalirudin in primary PCI for STEMI.
“The headline last year was that bivalirudin has a high stent thrombosis rate. It made the newspapers everywhere. But we never saw that, and we always thought that the difference might be in how we used the drug. There’s a new headline now, that this high stent thrombosis rate is not seen in clinical practice. The practice differs from the randomized trials, and the outcomes differ as well,” observed Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
In SCAAR, the 30-day rate of definite, angiographically proven stent thrombosis was 0.84% in 16,860 bivalirudin-treated patients, 0.94% in 3,182 who got heparin only, and 0.83% in 11,216 glycoprotein IIb/IIIa inhibitor recipients. These numeric differences weren’t statistically significant.
All-cause mortality 1 year post-PCI was 9.1% in patients with no stent thrombosis, 16.1% in those who experienced stent thrombosis within 1 day post PCI, and 23.0% in those whose stent thrombosis occurred on days 2-30. Dr. Grimfjard speculated that the explanation for the numerically higher 1-year all-cause mortality rate in patients whose stent thrombosis occurred on days 2-30 as opposed to day 0-1 is probably that they were more likely to have left the hospital when stent thrombosis occurred. That would translate to a longer time to repeat revascularization, hence a larger MI, more heart failure and arrhythmia, and thus a higher long-term risk of death.
Several audience members commented that they weren’t sure what to make of the observational Swedish data because of the looming presence of several potential confounders. For one, clinical practice trends changed considerably during the 7-year time frame of the study, as evidenced by the fact that the use of drug-eluting stents was far more common in bivalirudin-treated patients than in the glycoprotein IIb/IIIa inhibitor group. Also, Swedish cardiologists who put their STEMI patients on bivalirudin were more likely to utilize the more modern radial artery access in performing primary PCI; their practice may have differed from their colleagues’ in other, unrecorded ways as well, it was noted.
Dr. Grimfjard reported having no financial conflicts regarding the study, which was conducted free of commercial support.
AT EUROPCR 2015
Key clinical point: The 30-day incidence of stent thrombosis following primary PCI in a large, real-world STEMI population was reassuringly low regardless of the antithrombotic regimen.
Major finding: The stent thrombosis rate within 30 days after primary PCI for STEMI was 0.84% in patients who received bivalirudin for antithrombotic therapy, 0.94% with heparin only, and 0.83% with a glycoprotein IIb/IIIa inhibitor in this real-world nationwide Swedish registry.
Data source: A prospective observational cohort study which included all patients who underwent primary PCI for STEMI in Sweden during 2007-2014.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted free of commercial support.
Novel BioFreedom coronary stent looks promising
PARIS – A unique polymer- and carrier-free drug-eluting stent demonstrated a highly desirable early healing profile and a mere 4% target vessel revascularization rate at 9 months of follow-up in a 100-patient study presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This study is a proof of concept of the polymer-free approach. The stent retains neointimal suppression and antiproliferative efficacy like any other drug-eluting stent. If supported by long-term clinical results, this concept could have a major impact on future new stent platform development,” said Dr. Stephen Lee, professor of cardiology at the University of Hong Kong.
The BioFreedom stent was designed to avoid the chronic inflammation caused by the polymers and drug carriers utilized in other drug-eluting stents (DES), which necessitates long-term dual antiplatelet therapy (DAPT). The novel stent is a bare metal stent whose outer surface of stainless steel contains microstructural crevasses harboring a proprietary antirestenosis drug known as Biolimus A9. This is a highly lipophilic agent and hence has a strong affinity for the vessel wall. All of the drug elutes into the arterial tissue within 1 month, at which point the BioFreedom device becomes a bare metal stent – the type of stent that doesn’t require prolonged DAPT, with its attendant increased bleeding risks, Dr. Lee explained.
The 100-patient study, featuring a 100% follow-up rate at 12 months, utilized optical coherence tomography to assess the primary endpoint, which was stent strut coverage through 9 months as an indicator of early healing post intervention. At 1 month post-PCI, 85.8% of all struts were covered, as were 96.8% at 4 months, 97.1% at 5 months, and 99.6% at 9 months.
Neointimal thickness as assessed in a core laboratory increased minimally and slowly, confirming the drug’s antiproliferative effect. Neointimal thickness was 0.04 mm at 1 month, 0.06 mm at 5 months, and 1 mm at 9 months.
“This is the most important message of the study,” according to Dr. Lee. “It confirms the efficacy of the BioFreedom stent as a DES. If it didn’t curb neointimal thickness and just acted like a bare metal stent, there would be no point in using this mechanism.”
In-stent neointimal volume increased from 4.3% at 1 month to 13% at 9 months, which is “still very low” compared to other stent types, he added.
Four of 100 patients experienced asymptomatic in-stent restenosis requiring intervention within the first 9 months. With a mean follow-up duration of 468 days, there have been no additional cases of in-stent restenosis requiring treatment, no cases of late stent thrombosis, and indeed no other major adverse cardiovascular events.
Now well underway is a vastly larger clinical trial designed to confirm that the BioFreedom stent is as safe as a bare metal stent in patients at high risk of bleeding, while at the same time delivering the antirestenosis benefits of a DES. The LEADERS FREE trial is a 2,466-patient, prospective, randomized, double-blind clinical trial employing a 1-month course of DAPT following implantation of the BioFreedom stent or a bare metal stent. Results are anticipated late this year.
Dr. Lee received a research grant from Biosensors International, which sponsored the proof-of-concept study and is funding the LEADERS FREE trial.
PARIS – A unique polymer- and carrier-free drug-eluting stent demonstrated a highly desirable early healing profile and a mere 4% target vessel revascularization rate at 9 months of follow-up in a 100-patient study presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This study is a proof of concept of the polymer-free approach. The stent retains neointimal suppression and antiproliferative efficacy like any other drug-eluting stent. If supported by long-term clinical results, this concept could have a major impact on future new stent platform development,” said Dr. Stephen Lee, professor of cardiology at the University of Hong Kong.
The BioFreedom stent was designed to avoid the chronic inflammation caused by the polymers and drug carriers utilized in other drug-eluting stents (DES), which necessitates long-term dual antiplatelet therapy (DAPT). The novel stent is a bare metal stent whose outer surface of stainless steel contains microstructural crevasses harboring a proprietary antirestenosis drug known as Biolimus A9. This is a highly lipophilic agent and hence has a strong affinity for the vessel wall. All of the drug elutes into the arterial tissue within 1 month, at which point the BioFreedom device becomes a bare metal stent – the type of stent that doesn’t require prolonged DAPT, with its attendant increased bleeding risks, Dr. Lee explained.
The 100-patient study, featuring a 100% follow-up rate at 12 months, utilized optical coherence tomography to assess the primary endpoint, which was stent strut coverage through 9 months as an indicator of early healing post intervention. At 1 month post-PCI, 85.8% of all struts were covered, as were 96.8% at 4 months, 97.1% at 5 months, and 99.6% at 9 months.
Neointimal thickness as assessed in a core laboratory increased minimally and slowly, confirming the drug’s antiproliferative effect. Neointimal thickness was 0.04 mm at 1 month, 0.06 mm at 5 months, and 1 mm at 9 months.
“This is the most important message of the study,” according to Dr. Lee. “It confirms the efficacy of the BioFreedom stent as a DES. If it didn’t curb neointimal thickness and just acted like a bare metal stent, there would be no point in using this mechanism.”
In-stent neointimal volume increased from 4.3% at 1 month to 13% at 9 months, which is “still very low” compared to other stent types, he added.
Four of 100 patients experienced asymptomatic in-stent restenosis requiring intervention within the first 9 months. With a mean follow-up duration of 468 days, there have been no additional cases of in-stent restenosis requiring treatment, no cases of late stent thrombosis, and indeed no other major adverse cardiovascular events.
Now well underway is a vastly larger clinical trial designed to confirm that the BioFreedom stent is as safe as a bare metal stent in patients at high risk of bleeding, while at the same time delivering the antirestenosis benefits of a DES. The LEADERS FREE trial is a 2,466-patient, prospective, randomized, double-blind clinical trial employing a 1-month course of DAPT following implantation of the BioFreedom stent or a bare metal stent. Results are anticipated late this year.
Dr. Lee received a research grant from Biosensors International, which sponsored the proof-of-concept study and is funding the LEADERS FREE trial.
PARIS – A unique polymer- and carrier-free drug-eluting stent demonstrated a highly desirable early healing profile and a mere 4% target vessel revascularization rate at 9 months of follow-up in a 100-patient study presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This study is a proof of concept of the polymer-free approach. The stent retains neointimal suppression and antiproliferative efficacy like any other drug-eluting stent. If supported by long-term clinical results, this concept could have a major impact on future new stent platform development,” said Dr. Stephen Lee, professor of cardiology at the University of Hong Kong.
The BioFreedom stent was designed to avoid the chronic inflammation caused by the polymers and drug carriers utilized in other drug-eluting stents (DES), which necessitates long-term dual antiplatelet therapy (DAPT). The novel stent is a bare metal stent whose outer surface of stainless steel contains microstructural crevasses harboring a proprietary antirestenosis drug known as Biolimus A9. This is a highly lipophilic agent and hence has a strong affinity for the vessel wall. All of the drug elutes into the arterial tissue within 1 month, at which point the BioFreedom device becomes a bare metal stent – the type of stent that doesn’t require prolonged DAPT, with its attendant increased bleeding risks, Dr. Lee explained.
The 100-patient study, featuring a 100% follow-up rate at 12 months, utilized optical coherence tomography to assess the primary endpoint, which was stent strut coverage through 9 months as an indicator of early healing post intervention. At 1 month post-PCI, 85.8% of all struts were covered, as were 96.8% at 4 months, 97.1% at 5 months, and 99.6% at 9 months.
Neointimal thickness as assessed in a core laboratory increased minimally and slowly, confirming the drug’s antiproliferative effect. Neointimal thickness was 0.04 mm at 1 month, 0.06 mm at 5 months, and 1 mm at 9 months.
“This is the most important message of the study,” according to Dr. Lee. “It confirms the efficacy of the BioFreedom stent as a DES. If it didn’t curb neointimal thickness and just acted like a bare metal stent, there would be no point in using this mechanism.”
In-stent neointimal volume increased from 4.3% at 1 month to 13% at 9 months, which is “still very low” compared to other stent types, he added.
Four of 100 patients experienced asymptomatic in-stent restenosis requiring intervention within the first 9 months. With a mean follow-up duration of 468 days, there have been no additional cases of in-stent restenosis requiring treatment, no cases of late stent thrombosis, and indeed no other major adverse cardiovascular events.
Now well underway is a vastly larger clinical trial designed to confirm that the BioFreedom stent is as safe as a bare metal stent in patients at high risk of bleeding, while at the same time delivering the antirestenosis benefits of a DES. The LEADERS FREE trial is a 2,466-patient, prospective, randomized, double-blind clinical trial employing a 1-month course of DAPT following implantation of the BioFreedom stent or a bare metal stent. Results are anticipated late this year.
Dr. Lee received a research grant from Biosensors International, which sponsored the proof-of-concept study and is funding the LEADERS FREE trial.
AT EUROPCR 2015
Key clinical point: A novel coronary stent combines the antirestenotic benefits of a drug-eluting stent with the safety of a bare metal stent.
Major finding: One month after placement of the novel polymer- and carrier-free BioFreedom stainless steel DES, 85.8% of all struts were covered, as were 96.8% of struts at 4 months and 99.6% at 9 months.
Data source: This was a prospective proof-of-concept study in which 100 consecutive patients received the novel BioFreedom stent and underwent serial assessment of strut coverage by optical coherence tomography to document early healing.
Disclosures: The study was sponsored by Biosensors International, which provided the presenter with a research grant.
In PCI, switching clopidogrel nonresponders to prasugrel halved 2-year cardiac mortality
PARIS – Clopidogrel nonresponsiveness is a modifiable cardiovascular risk factor in patients undergoing percutaneous coronary intervention, according to the results of the third Responsiveness to Clopidogrel and Stent-Related Events (RECLOSE-3) study.
High residual platelet activity following a loading dose of clopidogrel in patients undergoing PCI was shown in the earlier RECLOSE-2 study to be a potent predictor of an increased 2-year cardiovascular event rate (JAMA 2011;306:1215-23). This left open the question of whether switching to a different antiplatelet drug would reduce that elevated 2-year risk.
The new RECLOSE-3 study shows that this clopidogrel nonresponsiveness is indeed a modifiable risk factor. All that’s necessary is to identify affected patients via a commercially available in vitro assay, switch them to prasugrel, and their long-term cardiac outcomes become markedly better than if they stayed on clopidogrel, Dr. David Antoniucci reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The prospective RECLOSE-3 study included 302 consecutive patients undergoing PCI who were determined to be clopidogrel nonresponders based upon residual platelet activity of 70% or more as measured by light transmittance aggregometry. All were switched to prasugrel and underwent repeat platelet activity measurement. The control group consisted of 248 clopidogrel nonresponders who stayed on the antiplatelet agent in RECLOSE-2.
It was necessary to rely on historical controls for ethical reasons; based upon the RECLOSE-2 results, it’s no longer appropriate to randomize clopidogrel nonresponders to continued use of clopidogrel, according to Dr. Antoniucci, head of the division of cardiology at Careggi Hospital in Florence, Italy.
Mean residual platelet reactivity improved from 78% in RECLOSE-3 participants on clopidogrel to 47% on prasugrel. All but 6% of clopidogrel nonresponders demonstrated acceptable suppression of platelet activity on prasugrel.
The primary study endpoint was 2-year cardiac mortality. With a follow-up rate of 99%, the rate was 4% in clopidogrel nonresponders switched to prasugrel, significantly better than the 9.7% in controls. Moreover, the rate of definite stent thrombosis – a key secondary endpoint – was 0.7% in the group switched to prasugrel, fourfold lower than in controls. Probable stent thrombosis was diagnosed in 1.6% of controls and none of the prasugrel group.
All patients in the control group from RECLOSE-2 had been admitted with an acute coronary syndrome. Restricting the analysis to the 126 RECLOSE-3 participants switched to prasugrel who had an acute coronary syndrome upon hospitalization, the 2-year cardiac death rate was 3.2%, still significantly lower than the 9.7% in controls.
In a multivariate analysis that controlled for potential confounders – including the more frequent use of drug-eluting stents and lower prevalence of a left ventricular ejection fraction of 40% or less in the RECLOSE-3 patients – switching clopidogrel nonresponders to prasugrel was associated with a highly significant 50% reduction in the risk of cardiac death at 2 years’ follow-up. The only other significant predictors were a baseline serum creatinine greater than 1.5 mg/dL and advanced age, both of which were associated with increased risk.
The RECLOSE-3 study was sponsored by the Italian Department of Health. Dr. Antoniucci reported having no financial conflicts.
bjancin@frontlinemedcom.com
PARIS – Clopidogrel nonresponsiveness is a modifiable cardiovascular risk factor in patients undergoing percutaneous coronary intervention, according to the results of the third Responsiveness to Clopidogrel and Stent-Related Events (RECLOSE-3) study.
High residual platelet activity following a loading dose of clopidogrel in patients undergoing PCI was shown in the earlier RECLOSE-2 study to be a potent predictor of an increased 2-year cardiovascular event rate (JAMA 2011;306:1215-23). This left open the question of whether switching to a different antiplatelet drug would reduce that elevated 2-year risk.
The new RECLOSE-3 study shows that this clopidogrel nonresponsiveness is indeed a modifiable risk factor. All that’s necessary is to identify affected patients via a commercially available in vitro assay, switch them to prasugrel, and their long-term cardiac outcomes become markedly better than if they stayed on clopidogrel, Dr. David Antoniucci reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The prospective RECLOSE-3 study included 302 consecutive patients undergoing PCI who were determined to be clopidogrel nonresponders based upon residual platelet activity of 70% or more as measured by light transmittance aggregometry. All were switched to prasugrel and underwent repeat platelet activity measurement. The control group consisted of 248 clopidogrel nonresponders who stayed on the antiplatelet agent in RECLOSE-2.
It was necessary to rely on historical controls for ethical reasons; based upon the RECLOSE-2 results, it’s no longer appropriate to randomize clopidogrel nonresponders to continued use of clopidogrel, according to Dr. Antoniucci, head of the division of cardiology at Careggi Hospital in Florence, Italy.
Mean residual platelet reactivity improved from 78% in RECLOSE-3 participants on clopidogrel to 47% on prasugrel. All but 6% of clopidogrel nonresponders demonstrated acceptable suppression of platelet activity on prasugrel.
The primary study endpoint was 2-year cardiac mortality. With a follow-up rate of 99%, the rate was 4% in clopidogrel nonresponders switched to prasugrel, significantly better than the 9.7% in controls. Moreover, the rate of definite stent thrombosis – a key secondary endpoint – was 0.7% in the group switched to prasugrel, fourfold lower than in controls. Probable stent thrombosis was diagnosed in 1.6% of controls and none of the prasugrel group.
All patients in the control group from RECLOSE-2 had been admitted with an acute coronary syndrome. Restricting the analysis to the 126 RECLOSE-3 participants switched to prasugrel who had an acute coronary syndrome upon hospitalization, the 2-year cardiac death rate was 3.2%, still significantly lower than the 9.7% in controls.
In a multivariate analysis that controlled for potential confounders – including the more frequent use of drug-eluting stents and lower prevalence of a left ventricular ejection fraction of 40% or less in the RECLOSE-3 patients – switching clopidogrel nonresponders to prasugrel was associated with a highly significant 50% reduction in the risk of cardiac death at 2 years’ follow-up. The only other significant predictors were a baseline serum creatinine greater than 1.5 mg/dL and advanced age, both of which were associated with increased risk.
The RECLOSE-3 study was sponsored by the Italian Department of Health. Dr. Antoniucci reported having no financial conflicts.
bjancin@frontlinemedcom.com
PARIS – Clopidogrel nonresponsiveness is a modifiable cardiovascular risk factor in patients undergoing percutaneous coronary intervention, according to the results of the third Responsiveness to Clopidogrel and Stent-Related Events (RECLOSE-3) study.
High residual platelet activity following a loading dose of clopidogrel in patients undergoing PCI was shown in the earlier RECLOSE-2 study to be a potent predictor of an increased 2-year cardiovascular event rate (JAMA 2011;306:1215-23). This left open the question of whether switching to a different antiplatelet drug would reduce that elevated 2-year risk.
The new RECLOSE-3 study shows that this clopidogrel nonresponsiveness is indeed a modifiable risk factor. All that’s necessary is to identify affected patients via a commercially available in vitro assay, switch them to prasugrel, and their long-term cardiac outcomes become markedly better than if they stayed on clopidogrel, Dr. David Antoniucci reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The prospective RECLOSE-3 study included 302 consecutive patients undergoing PCI who were determined to be clopidogrel nonresponders based upon residual platelet activity of 70% or more as measured by light transmittance aggregometry. All were switched to prasugrel and underwent repeat platelet activity measurement. The control group consisted of 248 clopidogrel nonresponders who stayed on the antiplatelet agent in RECLOSE-2.
It was necessary to rely on historical controls for ethical reasons; based upon the RECLOSE-2 results, it’s no longer appropriate to randomize clopidogrel nonresponders to continued use of clopidogrel, according to Dr. Antoniucci, head of the division of cardiology at Careggi Hospital in Florence, Italy.
Mean residual platelet reactivity improved from 78% in RECLOSE-3 participants on clopidogrel to 47% on prasugrel. All but 6% of clopidogrel nonresponders demonstrated acceptable suppression of platelet activity on prasugrel.
The primary study endpoint was 2-year cardiac mortality. With a follow-up rate of 99%, the rate was 4% in clopidogrel nonresponders switched to prasugrel, significantly better than the 9.7% in controls. Moreover, the rate of definite stent thrombosis – a key secondary endpoint – was 0.7% in the group switched to prasugrel, fourfold lower than in controls. Probable stent thrombosis was diagnosed in 1.6% of controls and none of the prasugrel group.
All patients in the control group from RECLOSE-2 had been admitted with an acute coronary syndrome. Restricting the analysis to the 126 RECLOSE-3 participants switched to prasugrel who had an acute coronary syndrome upon hospitalization, the 2-year cardiac death rate was 3.2%, still significantly lower than the 9.7% in controls.
In a multivariate analysis that controlled for potential confounders – including the more frequent use of drug-eluting stents and lower prevalence of a left ventricular ejection fraction of 40% or less in the RECLOSE-3 patients – switching clopidogrel nonresponders to prasugrel was associated with a highly significant 50% reduction in the risk of cardiac death at 2 years’ follow-up. The only other significant predictors were a baseline serum creatinine greater than 1.5 mg/dL and advanced age, both of which were associated with increased risk.
The RECLOSE-3 study was sponsored by the Italian Department of Health. Dr. Antoniucci reported having no financial conflicts.
bjancin@frontlinemedcom.com
AT EuroPCR 2015
Key clinical point: Switch clopidogrel nonresponders undergoing PCI to prasugrel in order to cut their 2-year cardiac mortality risk in half.
Major finding: Patients undergoing PCI who switch to prasugrel because they show high residual platelet activity after a loading dose of clopidogrel have a 2-year cardiac mortality half that of clopidogrel nonresponders who remain on the drug.
Data source: RECLOSE-3, a prospective study of 302 patients undergoing PCI who were switched to prasugrel upon being identified as clopidogrel nonresponders and a historical control group of 248 clopidogrel nonresponders who remained on the antiplatelet agent.
Disclosures: RECLOSE-3 was funded by the Italian Department of Health. The presenter reported having no financial conflicts.
Contrast injection approximates adenosine-derived FFR
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
AT EUROPCR 2015
Key clinical point: Intracoronary injection of contrast media to achieve hyperemia is a safe, simple, and inexpensive alternative to adenosine-derived fractional flow reserve in assessing the functional significance of coronary stenoses.
Major finding: When a stenotic lesion’s Pd/Pa is 0.8 or less as measured during hyperemia induced by an intracoronary injection of contrast media, it’s 0.8 or less according to adenosine-derived FFR as well.
Data source: CONTRAST, a prospective, 750-patient, nine-country diagnostic accuracy study in which the same coronary stenoses were evaluated multiple times using standard adenosine-based FFR as well as measurements obtained under hyperemia induced by an intracoronary contrast media injection, at physiologic rest, and using instantaneous wave-free rate technology.
Disclosures: The study was sponsored by the University of Texas Medical Center at Houston. The presenter reported receiving a research grant from St. Jude Medical to conduct the study.
Real-time detection of aortic regurgitation during TAVI without TEE
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
AT EUROPCR
Key clinical point: A real-time assay for defects in von Willebrand factor may enable most patients undergoing transcatheter aortic valve replacement to avoid transesophageal echocardiography under general anesthesia in order to assess for clinically significant aortic regurgitation following valve replacement.
Major finding: An assay for PFA-CADP identifies patients who have more than mild aortic regurgitation immediately after valve implantation, while they are still in the cath lab and can readily undergo dilation with a larger balloon in order to fix the problem.
Data source: This ongoing study includes to date 88 consecutive patients undergoing transcatheter aortic valve implantation via the transfemoral route.
Disclosures: This study is sponsored by Lille (France) University. The presenter reported having no financial conflicts.
Reports of TAVI leaflet thickening downplayed – for now
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
EXPERT ANALYSIS FROM EUROPCR
Erectile dysfunction: New frontier in interventional cardiology
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
AT EUROPCR 2015
Key clinical point: Balloon angioplasty of penile stenoses was an effective treatment for erectile dysfunction in the majority of treated patients.
Major finding: Twelve of 22 men with erectile dysfunction who underwent balloon angioplasty for penile artery stenoses had clinically meaningful improvement in erectile function at 12 months of follow-up.
Data source: Prospective study of 22 patients with erectile dysfunction.
Disclosures: The study was conducted without commercial support and the presenter reported having no financial conflicts.




















