User login
EuroPCR 2015
New tools aid decisions on length of dual-antiplatelet therapy
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
AT EuroPCR 2015
Key clinical point: Stent location and CRUSADE score can inform decisions about the duration of dual-antiplatelet therapy.
Major finding: Coronary stent recipients with a CRUSADE bleeding risk score above 40 had a 2.7-fold greater risk of a major bleeding event if randomized to 24 months rather than 6 months of dual-antiplatelet therapy.
Data source: A retrospective, hypothesis-generating secondary analysis of the 2,103-patient prospective randomized PRODIGY study.
Disclosures: This analysis was conducted without external funding. The presenter reported having no relevant financial conflicts.
European cardiologists seek involvement in acute stroke
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
EXPERT ANALYSIS FROM EUROPCR 2015
EuroPCR: OCT stenting guidance may decrease MIs
PARIS – Optical coherence tomography guidance of percutaneous coronary intervention resulted in a change in PCI strategy in two-thirds of patients in the multicenter ILUMIEN I study.
“We were surprised by the high rate at which the OCT [optical coherence tomography] findings influenced practice. Physician decision making was influenced by OCT findings pre-PCI and/or post PCI in 65% of patients, mostly those with more complex disease,” Dr. William Wijns reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Another unexpected finding: The acute MI rate through 1 year of follow-up was zero among patients whose cardiologists altered their initial stenting strategy in response to the pre-PCI OCT findings and then performed post-PCI stent optimization because they deemed the initial deployment unacceptable based upon the post-PCI OCT findings.
In contrast, the MI rates were 10.3%-13.2% when cardiologists didn’t alter their strategy in response to either of the OCT results or when they altered it only once, based upon either the pre- or post-PCI OCT images. The great majority of these MIs occurred periprocedurally.
“These were true MIs with symptoms, not just enzyme bumps. The reduced MI rate in the subgroup of patients in whom operators changed the procedure based on OCT data, both pre- and post-PCI, was a surprise. The more you work on the artery, the more you’d expect to have troponin increases, at least,” observed Dr. Wijns of the cardiovascular center at Aalst, Belgium, and principal investigator in ILUMIEN I.
He was quick to add that the observed disparity in MI rates based upon the extent to which interventional cardiologists acted upon OCT findings was the result of a post hoc analysis and therefore must be considered merely hypothesis generating. It is, however, an exciting hypothesis, and one which will be tested prospectively in future randomized trials.
ILUMIEN I was a 40-center, 418-patient, prospective, randomized, observational study conducted in the United States, Europe, and Asia. The purpose of the study was to learn what impact OCT imaging had on procedural technique and to identify OCT findings that predict clinical outcomes.
All participants underwent paired fractional flow reserve and OCT studies at the time of angiography prior to their planned PCI and once again immediately post PCI. If the post-PCI imaging showed a suboptimal initial result – stent underexpansion with greater than 20% in-stent residual diameter stenosis, malapposition, flow-limiting edge dissection, or thrombus and/or tissue protrusion causing flow reduction – cardiologists had the option of optimizing the results. If they elected to do so, then OCT imaging was performed once again post optimization to see if in fact the technical outcomes had been improved as assessed in a core laboratory.
Pre-PCI measurements of fractional flow reserve and OCT were successfully accomplished in 91% and 98% of patients, respectively. Armed with the fractional flow reserve data, the interventional cardiologists developed their initial PCI strategy. Then they received the OCT results. Based upon these preprocedural OCT findings, cardiologists changed their PCI strategy in 57% of cases.
Post-PCI fractional flow reserve and OCT results were acquired in 83% and 98% of patients, respectively. Based upon what interventionalists saw as an unacceptable initial PCI result apparent upon the second OCT findings, they performed PCI optimization in 27% of patients.
OCT is known to have superior resolution, compared with angiography or, for that matter, intravascular ultrasound, so it’s not surprising that analysis of the post-PCI OCT findings at the central core laboratory identified a high rate of abnormal findings following what interventionalists deemed a successful result based upon angiographic appearance. Malapposition was present in 32% of cases, stent underexpansion in 27%, edge dissection in 32%, malapposition plus edge dissection in 9%, and tissue or thrombus protrusion in 4%.
Cardiologists performed PCI optimization based upon the second OCT findings in 106 patients. The third and final round of OCT in those patients showed that OCT-guided optimization achieved a sharp decrease in the rates of malapposition and malapposition plus edge dissection.
The 1-year major adverse cardiovascular event rate ranged from a low of 11.5% in the 65 patients who had a change in PCI strategy based upon the preprocedural OCT findings and who also underwent OCT-guided post-PCI optimization to 15.9% in the 137 patients who had neither. Stent thrombosis rates were very low in all four groups, as was in-hospital mortality.
Dr. Wijns noted that analysis of OCT guidance parameters predictive of 1-year clinical outcomes is ongoing.
The ILUMIEN I study was sponsored by St. Jude Medical. Dr. Wijns is a consultant to the company.
PARIS – Optical coherence tomography guidance of percutaneous coronary intervention resulted in a change in PCI strategy in two-thirds of patients in the multicenter ILUMIEN I study.
“We were surprised by the high rate at which the OCT [optical coherence tomography] findings influenced practice. Physician decision making was influenced by OCT findings pre-PCI and/or post PCI in 65% of patients, mostly those with more complex disease,” Dr. William Wijns reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Another unexpected finding: The acute MI rate through 1 year of follow-up was zero among patients whose cardiologists altered their initial stenting strategy in response to the pre-PCI OCT findings and then performed post-PCI stent optimization because they deemed the initial deployment unacceptable based upon the post-PCI OCT findings.
In contrast, the MI rates were 10.3%-13.2% when cardiologists didn’t alter their strategy in response to either of the OCT results or when they altered it only once, based upon either the pre- or post-PCI OCT images. The great majority of these MIs occurred periprocedurally.
“These were true MIs with symptoms, not just enzyme bumps. The reduced MI rate in the subgroup of patients in whom operators changed the procedure based on OCT data, both pre- and post-PCI, was a surprise. The more you work on the artery, the more you’d expect to have troponin increases, at least,” observed Dr. Wijns of the cardiovascular center at Aalst, Belgium, and principal investigator in ILUMIEN I.
He was quick to add that the observed disparity in MI rates based upon the extent to which interventional cardiologists acted upon OCT findings was the result of a post hoc analysis and therefore must be considered merely hypothesis generating. It is, however, an exciting hypothesis, and one which will be tested prospectively in future randomized trials.
ILUMIEN I was a 40-center, 418-patient, prospective, randomized, observational study conducted in the United States, Europe, and Asia. The purpose of the study was to learn what impact OCT imaging had on procedural technique and to identify OCT findings that predict clinical outcomes.
All participants underwent paired fractional flow reserve and OCT studies at the time of angiography prior to their planned PCI and once again immediately post PCI. If the post-PCI imaging showed a suboptimal initial result – stent underexpansion with greater than 20% in-stent residual diameter stenosis, malapposition, flow-limiting edge dissection, or thrombus and/or tissue protrusion causing flow reduction – cardiologists had the option of optimizing the results. If they elected to do so, then OCT imaging was performed once again post optimization to see if in fact the technical outcomes had been improved as assessed in a core laboratory.
Pre-PCI measurements of fractional flow reserve and OCT were successfully accomplished in 91% and 98% of patients, respectively. Armed with the fractional flow reserve data, the interventional cardiologists developed their initial PCI strategy. Then they received the OCT results. Based upon these preprocedural OCT findings, cardiologists changed their PCI strategy in 57% of cases.
Post-PCI fractional flow reserve and OCT results were acquired in 83% and 98% of patients, respectively. Based upon what interventionalists saw as an unacceptable initial PCI result apparent upon the second OCT findings, they performed PCI optimization in 27% of patients.
OCT is known to have superior resolution, compared with angiography or, for that matter, intravascular ultrasound, so it’s not surprising that analysis of the post-PCI OCT findings at the central core laboratory identified a high rate of abnormal findings following what interventionalists deemed a successful result based upon angiographic appearance. Malapposition was present in 32% of cases, stent underexpansion in 27%, edge dissection in 32%, malapposition plus edge dissection in 9%, and tissue or thrombus protrusion in 4%.
Cardiologists performed PCI optimization based upon the second OCT findings in 106 patients. The third and final round of OCT in those patients showed that OCT-guided optimization achieved a sharp decrease in the rates of malapposition and malapposition plus edge dissection.
The 1-year major adverse cardiovascular event rate ranged from a low of 11.5% in the 65 patients who had a change in PCI strategy based upon the preprocedural OCT findings and who also underwent OCT-guided post-PCI optimization to 15.9% in the 137 patients who had neither. Stent thrombosis rates were very low in all four groups, as was in-hospital mortality.
Dr. Wijns noted that analysis of OCT guidance parameters predictive of 1-year clinical outcomes is ongoing.
The ILUMIEN I study was sponsored by St. Jude Medical. Dr. Wijns is a consultant to the company.
PARIS – Optical coherence tomography guidance of percutaneous coronary intervention resulted in a change in PCI strategy in two-thirds of patients in the multicenter ILUMIEN I study.
“We were surprised by the high rate at which the OCT [optical coherence tomography] findings influenced practice. Physician decision making was influenced by OCT findings pre-PCI and/or post PCI in 65% of patients, mostly those with more complex disease,” Dr. William Wijns reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Another unexpected finding: The acute MI rate through 1 year of follow-up was zero among patients whose cardiologists altered their initial stenting strategy in response to the pre-PCI OCT findings and then performed post-PCI stent optimization because they deemed the initial deployment unacceptable based upon the post-PCI OCT findings.
In contrast, the MI rates were 10.3%-13.2% when cardiologists didn’t alter their strategy in response to either of the OCT results or when they altered it only once, based upon either the pre- or post-PCI OCT images. The great majority of these MIs occurred periprocedurally.
“These were true MIs with symptoms, not just enzyme bumps. The reduced MI rate in the subgroup of patients in whom operators changed the procedure based on OCT data, both pre- and post-PCI, was a surprise. The more you work on the artery, the more you’d expect to have troponin increases, at least,” observed Dr. Wijns of the cardiovascular center at Aalst, Belgium, and principal investigator in ILUMIEN I.
He was quick to add that the observed disparity in MI rates based upon the extent to which interventional cardiologists acted upon OCT findings was the result of a post hoc analysis and therefore must be considered merely hypothesis generating. It is, however, an exciting hypothesis, and one which will be tested prospectively in future randomized trials.
ILUMIEN I was a 40-center, 418-patient, prospective, randomized, observational study conducted in the United States, Europe, and Asia. The purpose of the study was to learn what impact OCT imaging had on procedural technique and to identify OCT findings that predict clinical outcomes.
All participants underwent paired fractional flow reserve and OCT studies at the time of angiography prior to their planned PCI and once again immediately post PCI. If the post-PCI imaging showed a suboptimal initial result – stent underexpansion with greater than 20% in-stent residual diameter stenosis, malapposition, flow-limiting edge dissection, or thrombus and/or tissue protrusion causing flow reduction – cardiologists had the option of optimizing the results. If they elected to do so, then OCT imaging was performed once again post optimization to see if in fact the technical outcomes had been improved as assessed in a core laboratory.
Pre-PCI measurements of fractional flow reserve and OCT were successfully accomplished in 91% and 98% of patients, respectively. Armed with the fractional flow reserve data, the interventional cardiologists developed their initial PCI strategy. Then they received the OCT results. Based upon these preprocedural OCT findings, cardiologists changed their PCI strategy in 57% of cases.
Post-PCI fractional flow reserve and OCT results were acquired in 83% and 98% of patients, respectively. Based upon what interventionalists saw as an unacceptable initial PCI result apparent upon the second OCT findings, they performed PCI optimization in 27% of patients.
OCT is known to have superior resolution, compared with angiography or, for that matter, intravascular ultrasound, so it’s not surprising that analysis of the post-PCI OCT findings at the central core laboratory identified a high rate of abnormal findings following what interventionalists deemed a successful result based upon angiographic appearance. Malapposition was present in 32% of cases, stent underexpansion in 27%, edge dissection in 32%, malapposition plus edge dissection in 9%, and tissue or thrombus protrusion in 4%.
Cardiologists performed PCI optimization based upon the second OCT findings in 106 patients. The third and final round of OCT in those patients showed that OCT-guided optimization achieved a sharp decrease in the rates of malapposition and malapposition plus edge dissection.
The 1-year major adverse cardiovascular event rate ranged from a low of 11.5% in the 65 patients who had a change in PCI strategy based upon the preprocedural OCT findings and who also underwent OCT-guided post-PCI optimization to 15.9% in the 137 patients who had neither. Stent thrombosis rates were very low in all four groups, as was in-hospital mortality.
Dr. Wijns noted that analysis of OCT guidance parameters predictive of 1-year clinical outcomes is ongoing.
The ILUMIEN I study was sponsored by St. Jude Medical. Dr. Wijns is a consultant to the company.
AT EUROPCR 2015
Key clinical point: OCT findings result in a change in PCI strategy in the majority of patients undergoing coronary stenting.
Major finding: OCT imaging results obtained pre- and post PCI altered interventional cardiologists’ PCI strategy in 65% of treated patients.
Data source: This was a three-continent, 40-center, 418-patient, prospective, nonrandomized, observational study.
Disclosures: The ILUMIEN I study was sponsored by St. Jude Medical. The presenter is a consultant to the company.
EuroPCR: New Sapien 3 TAVI valve findings show ‘wow’ factor
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
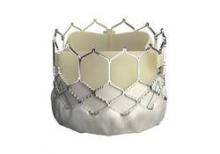
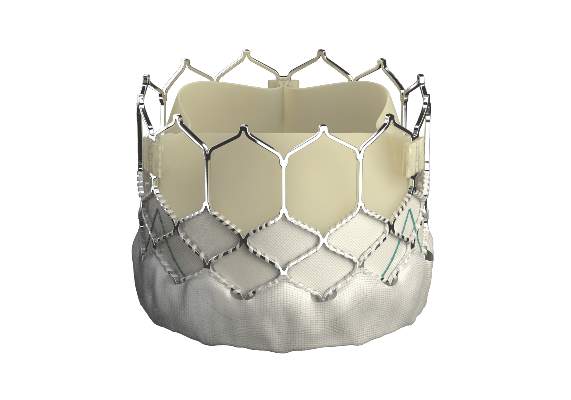
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
AT EUROPCR 2015
Key clinical point: Short-term outcomes following transcatheter aortic valve implantation using the Sapien 3 valve in patients with severe aortic stenosis at intermediate surgical risk are the best ever reported with TAVI or surgical valve replacement.
Major finding: Key 30-day outcomes after TAVI using the Sapien 3 valve via a transfemoral approach in intermediate–surgical risk patients with severe aortic stenosis included 1% overall mortality, a 2% rate of disabling stroke, and no severe paravalvular aortic regurgitation.
Data source: The European Sapien 3 CE IR study is a nonrandomized study of 101 intermediate–surgical risk octogenarians with severe aortic stenosis who underwent TAVI with the Sapien 3 valve via a transfemoral approach.
Disclosures: The European Sapien 3 CE IR study is sponsored by Edwards Lifesciences. The presenter is a consultant to the company.
EuroPCR: Which TAVI vascular closure device is safest?
PARIS – The Perclose ProGlide vascular closure device resulted in significantly fewer major vascular complications than its chief competitor, the Prostar XL, in patients undergoing transcatheter aortic valve implantation by a percutaneous transfemoral approach in the multicenter CONTROL trial.
“ProGlide-based vascular closure is associated with significantly lower rates of arterial rupture and hematomas, lower rates of major vascular complications, bleeding, acute kidney injury, and shorter hospital stay,” Dr. Israel M. Barbash reported in presenting the CONTROL results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Serious vascular complications remain a major concern when transcatheter aortic valve implantation (TAVI) is performed via a percutaneous transfemoral approach. The CONTROL study was conducted to determine whether the two closure devices most widely used for this purpose worldwide – the Prostar XL and Perclose ProGlide, both marketed by Abbott Vascular – differ in their adverse event rates.
CONTROL was a nine-center, international, retrospective, nonrandomized, matched-pairs comparison study. Starting with a pool of 3,138 percutaneous transfemoral TAVI patients, investigators used propensity score matching on nine variables to narrow the study population to 1,270 patients in 635 closely matched pairs.
The variables used in the matching process fell into three categories: comorbid conditions, including diabetes, coronary artery disease, and peripheral vascular disease; arterial factors such as tortuosity and calcification; and sheath type and size. Most patients underwent TAVI with a Cook Check-flo Performer or Edwards expandable eSheath and received a CoreValve or Sapien XT heart valve.
The major vascular complication rate was 2% in the ProGlide group, and more than threefold higher at roughly 7.5% in the Prostar group. Rates of life-threatening bleeding and major bleeding by the Valve Academic Research Consortium-2 (VARC-2) definitions also were significantly higher with the use of the Prostar device. In addition, rates of hematoma and femoral artery rupture were both threefold higher in the Prostar group.
Acute kidney injury occurred in 6.6% of the Prostar group, compared with 2.7% with ProGlide. The median hospital length of stay was a full day longer in the Prostar group: 6 days versus 5 days, according to Dr. Barbash of Sheba Medical Center in Tel Hashomer, Israel.
Despite the consistently higher adverse event rates documented in the Prostar group, in-hospital mortality rates didn’t differ between the two study arms. There was, however, a trend for lower in-hospital mortality in the ProGlide group by a margin of 3.5% versus 4.9% with Prostar; this difference might well have achieved statistical significance with larger patient numbers, the cardiologist continued.
A learning curve was evident for the ProGlide device: after an operator’s first 20 cases, the vascular complication rate dropped sharply. In contrast, the high adverse event rates associated with the Prostar device didn’t decrease significantly no matter how much experience with the device an operator gained.
“It looks like ‘Goodby Prostar, hello ProGlide.’ That’s what your data say to me,” said session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
Asked if he thinks a randomized trial is warranted in light of the clear and consistent messages provided by the CONTROL study, Dr. Barbash replied, yes, but not yet.
“Sheath sizes are decreasing and new players will come into the vascular closure device market soon. When they do, that will be the time for a randomized trial comparing the new ones to the older ones,” he added.
CONTROL was an investigator-initiated study. Dr. Barbash reported having no financial conflicts.
PARIS – The Perclose ProGlide vascular closure device resulted in significantly fewer major vascular complications than its chief competitor, the Prostar XL, in patients undergoing transcatheter aortic valve implantation by a percutaneous transfemoral approach in the multicenter CONTROL trial.
“ProGlide-based vascular closure is associated with significantly lower rates of arterial rupture and hematomas, lower rates of major vascular complications, bleeding, acute kidney injury, and shorter hospital stay,” Dr. Israel M. Barbash reported in presenting the CONTROL results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Serious vascular complications remain a major concern when transcatheter aortic valve implantation (TAVI) is performed via a percutaneous transfemoral approach. The CONTROL study was conducted to determine whether the two closure devices most widely used for this purpose worldwide – the Prostar XL and Perclose ProGlide, both marketed by Abbott Vascular – differ in their adverse event rates.
CONTROL was a nine-center, international, retrospective, nonrandomized, matched-pairs comparison study. Starting with a pool of 3,138 percutaneous transfemoral TAVI patients, investigators used propensity score matching on nine variables to narrow the study population to 1,270 patients in 635 closely matched pairs.
The variables used in the matching process fell into three categories: comorbid conditions, including diabetes, coronary artery disease, and peripheral vascular disease; arterial factors such as tortuosity and calcification; and sheath type and size. Most patients underwent TAVI with a Cook Check-flo Performer or Edwards expandable eSheath and received a CoreValve or Sapien XT heart valve.
The major vascular complication rate was 2% in the ProGlide group, and more than threefold higher at roughly 7.5% in the Prostar group. Rates of life-threatening bleeding and major bleeding by the Valve Academic Research Consortium-2 (VARC-2) definitions also were significantly higher with the use of the Prostar device. In addition, rates of hematoma and femoral artery rupture were both threefold higher in the Prostar group.
Acute kidney injury occurred in 6.6% of the Prostar group, compared with 2.7% with ProGlide. The median hospital length of stay was a full day longer in the Prostar group: 6 days versus 5 days, according to Dr. Barbash of Sheba Medical Center in Tel Hashomer, Israel.
Despite the consistently higher adverse event rates documented in the Prostar group, in-hospital mortality rates didn’t differ between the two study arms. There was, however, a trend for lower in-hospital mortality in the ProGlide group by a margin of 3.5% versus 4.9% with Prostar; this difference might well have achieved statistical significance with larger patient numbers, the cardiologist continued.
A learning curve was evident for the ProGlide device: after an operator’s first 20 cases, the vascular complication rate dropped sharply. In contrast, the high adverse event rates associated with the Prostar device didn’t decrease significantly no matter how much experience with the device an operator gained.
“It looks like ‘Goodby Prostar, hello ProGlide.’ That’s what your data say to me,” said session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
Asked if he thinks a randomized trial is warranted in light of the clear and consistent messages provided by the CONTROL study, Dr. Barbash replied, yes, but not yet.
“Sheath sizes are decreasing and new players will come into the vascular closure device market soon. When they do, that will be the time for a randomized trial comparing the new ones to the older ones,” he added.
CONTROL was an investigator-initiated study. Dr. Barbash reported having no financial conflicts.
PARIS – The Perclose ProGlide vascular closure device resulted in significantly fewer major vascular complications than its chief competitor, the Prostar XL, in patients undergoing transcatheter aortic valve implantation by a percutaneous transfemoral approach in the multicenter CONTROL trial.
“ProGlide-based vascular closure is associated with significantly lower rates of arterial rupture and hematomas, lower rates of major vascular complications, bleeding, acute kidney injury, and shorter hospital stay,” Dr. Israel M. Barbash reported in presenting the CONTROL results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Serious vascular complications remain a major concern when transcatheter aortic valve implantation (TAVI) is performed via a percutaneous transfemoral approach. The CONTROL study was conducted to determine whether the two closure devices most widely used for this purpose worldwide – the Prostar XL and Perclose ProGlide, both marketed by Abbott Vascular – differ in their adverse event rates.
CONTROL was a nine-center, international, retrospective, nonrandomized, matched-pairs comparison study. Starting with a pool of 3,138 percutaneous transfemoral TAVI patients, investigators used propensity score matching on nine variables to narrow the study population to 1,270 patients in 635 closely matched pairs.
The variables used in the matching process fell into three categories: comorbid conditions, including diabetes, coronary artery disease, and peripheral vascular disease; arterial factors such as tortuosity and calcification; and sheath type and size. Most patients underwent TAVI with a Cook Check-flo Performer or Edwards expandable eSheath and received a CoreValve or Sapien XT heart valve.
The major vascular complication rate was 2% in the ProGlide group, and more than threefold higher at roughly 7.5% in the Prostar group. Rates of life-threatening bleeding and major bleeding by the Valve Academic Research Consortium-2 (VARC-2) definitions also were significantly higher with the use of the Prostar device. In addition, rates of hematoma and femoral artery rupture were both threefold higher in the Prostar group.
Acute kidney injury occurred in 6.6% of the Prostar group, compared with 2.7% with ProGlide. The median hospital length of stay was a full day longer in the Prostar group: 6 days versus 5 days, according to Dr. Barbash of Sheba Medical Center in Tel Hashomer, Israel.
Despite the consistently higher adverse event rates documented in the Prostar group, in-hospital mortality rates didn’t differ between the two study arms. There was, however, a trend for lower in-hospital mortality in the ProGlide group by a margin of 3.5% versus 4.9% with Prostar; this difference might well have achieved statistical significance with larger patient numbers, the cardiologist continued.
A learning curve was evident for the ProGlide device: after an operator’s first 20 cases, the vascular complication rate dropped sharply. In contrast, the high adverse event rates associated with the Prostar device didn’t decrease significantly no matter how much experience with the device an operator gained.
“It looks like ‘Goodby Prostar, hello ProGlide.’ That’s what your data say to me,” said session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
Asked if he thinks a randomized trial is warranted in light of the clear and consistent messages provided by the CONTROL study, Dr. Barbash replied, yes, but not yet.
“Sheath sizes are decreasing and new players will come into the vascular closure device market soon. When they do, that will be the time for a randomized trial comparing the new ones to the older ones,” he added.
CONTROL was an investigator-initiated study. Dr. Barbash reported having no financial conflicts.
AT EuroPCR 2015
Key clinical point: Key outcomes are significantly better with the Perclose ProGlide vascular closure device than the Prostar XL in patients undergoing transcatheter aortic valve implantation via a percutaneous transfemoral approach.
Major finding: Patients who underwent transcatheter aortic valve implantation via a percutaneous transfemoral approach with vascular closure using the Perclose ProGlide device had fewer major vascular complications and a 1-day shorter hospital length of stay than with the Prostar XL closure device.
Data source: CONTROL was a retrospective, propensity score-matched comparison of outcomes in 635 pairs of patients. One patient in each pair was treated with the Perclose ProGlide vascular closure device, the other with the Prostar XL.
Disclosures: The CONTROL study was investigator initiated. The presenter reported having no financial conflicts.
TAVI embolic protection device shows favorable safety, efficacy
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
AT EUROPCR 2015
Key clinical point: Transcatheter aortic valve implantation has underappreciated adverse neurologic and cognitive consequences that can largely be avoided via an effective embolic protection device.
Major finding: Fifteen percent of patients showed new neurologic deficits on the NIH Stroke Scale after TAVI without cerebrovascular protection, a rate fivefold higher than in patients who used the TriGuard embolic protection device.
Data source: DEFLECT III, which randomized 85 participants to TAVI with the TriGuard embolic protection device or unprotected TAVI.
Disclosures: The DEFLECT III study was funded by Keystone Health. The presenter is on the company’s scientific advisory board.
Paclitaxel balloon achieves unprecedented success in complex PAD
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
AT EuroPCR
Key clinical point: A paclitaxel-coated angioplasty balloon results in 12-month patency rates never before seen in patients with peripheral arterial disease involving long lesions in the superficial femoral arteries.
Major finding: The 12-month primary patency rate was 91.1% in patients whose average lesion length was 26.4 cm.
Data source: This prospective, multinational study included 157 subjects with complex superficial femoral artery lesions at least 15 cm in length.
Disclosures: The IN.PACT Global Study is funded by Medtronic. The presenter is on the company’s scientific advisory board.
NOTION: TAVI has edge in patients at low surgical risk
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.
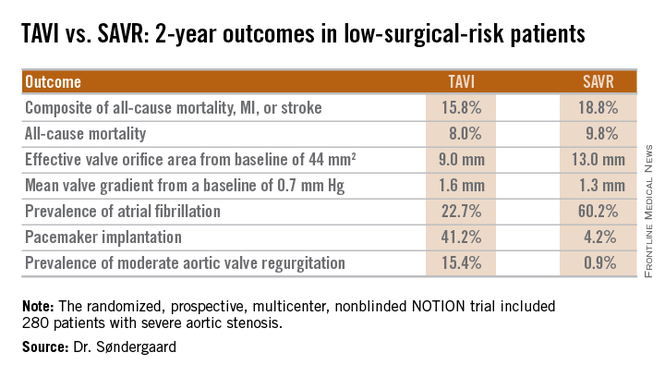
Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.

Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.

Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
AT EUROPCR
Key clinical point: At 2 years, outcomes of TAVI look as good as and in some domains better than outcomes of surgical aortic valve replacement in low-surgical-risk patients.
Major finding: The 2-year composite outcome of all-cause mortality, MI, or stroke occurred in 15.8% of the TAVI group compared with 18.8% of surgically treated patients.
Data source: The randomized, prospective, multicenter, nonblinded NOTION trial includes 280 low-surgical-risk patients with severe aortic stenosis.
Disclosures: The NOTION trial was sponsored by the Danish Heart Foundation. The presenter reported having no financial conflicts.
EuroPCR: CT-derived FFR promising in evaluating chest pain
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
AT EUROPCR
Key clinical point: Clinically decisive anatomic and physiologic data regarding the coronary arteries of patients with stable angina can be obtained noninvasively with a single test: CT-derived fractional flow reserve.
Major finding: Noninvasive FFR-CT findings resulted in a change in management strategy for 36% of patients with stable angina whose initial treatment plan was based on CT angiography alone.
Data source: A proof-of-concept study involving 200 patients with stable angina and a panel of three experienced interventional cardiologists making consensus decisions regarding their appropriate management.
Disclosures: The FFR-CT RIPCORD study was sponsored by Heartflow. The presenter reported having received research support from the company.