User login
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
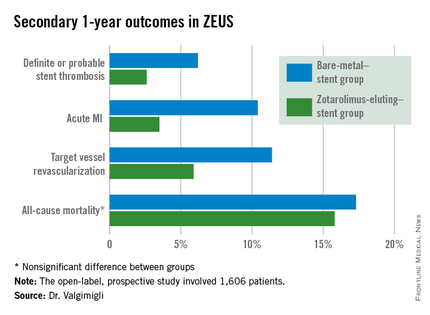
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.
Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
AT EUROPCR 2015
Key clinical point: High bleeding risk patients fare significantly better with a second-generation drug-eluting stent and 30 days of dual antiplatelet therapy than with a bare-metal stent.
Major finding: The 1-year incidence of major adverse cardiovascular events was 29.6% in high bleeding risk patients who received a bare-metal stent and 22.6% in those who got a second-generation zotarolimus-eluting stent with 30 days of dual antiplatelet therapy.
Data source: This was a prespecified analysis of 828 high bleeding risk patients randomized to a bare-metal stent or a second-generation zotarolimus-eluting stent in conjunction with 30 days of DAPT and then followed prospectively for 1 year.
Disclosures: The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. The presenter serves as a consultant and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.

