User login
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
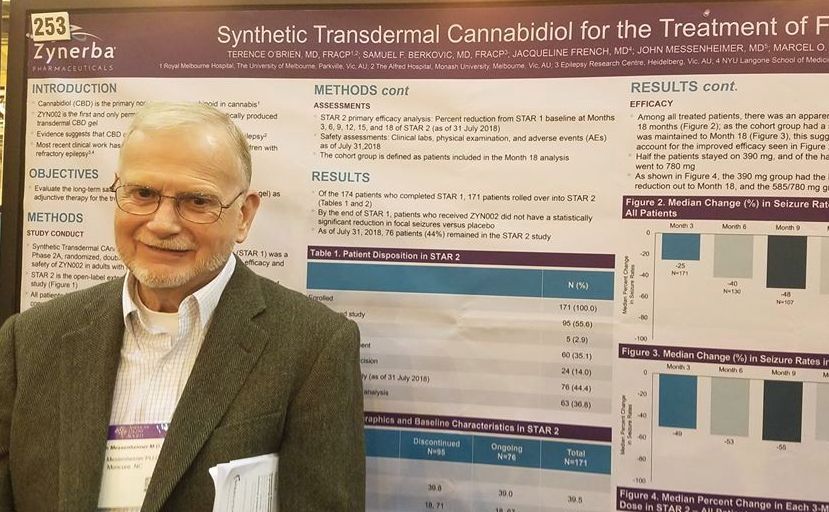
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
REPORTING FROM AES 2018
Key clinical point:
Major finding: The gel reduced uncontrolled focal seizures by a median of about 50%.
Study details: The open-label extension study comprised 171 subjects.
Disclosures: Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
Source: O’Brien TJ et al. AES 2018, Abstract 2.253