User login
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
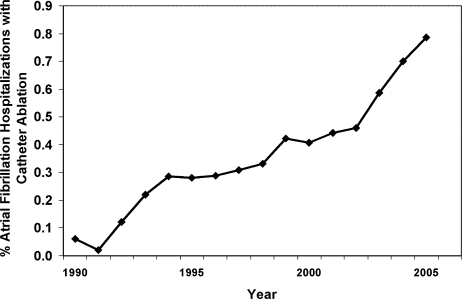
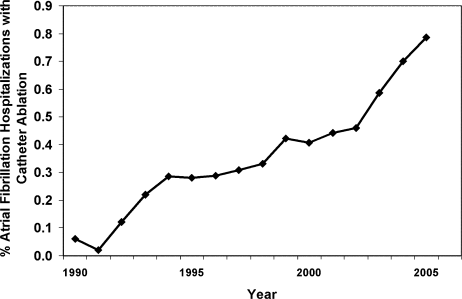
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).
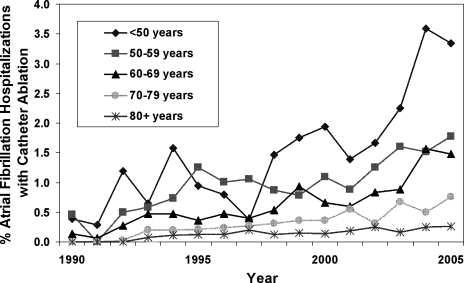
On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).
| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).

On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).

| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).

On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).

| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
Copyright © 2009 Society of Hospital Medicine