User login
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
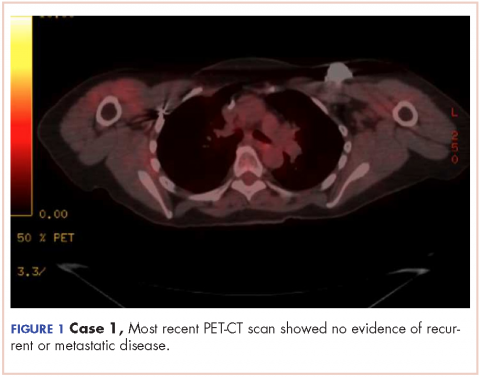
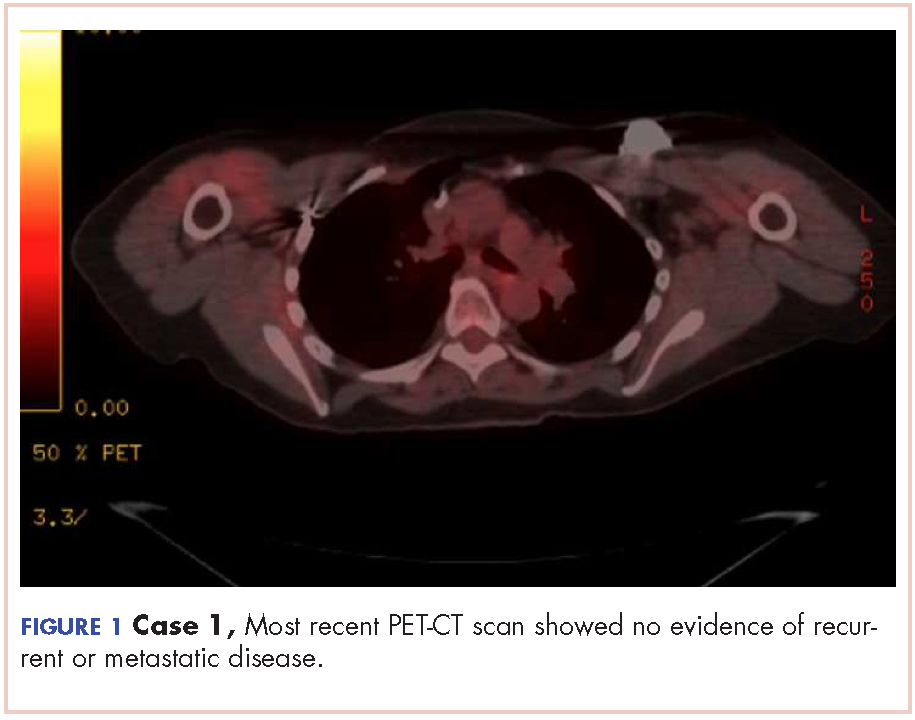
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
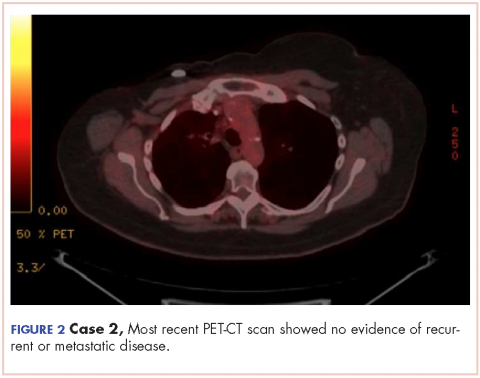
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.