User login
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
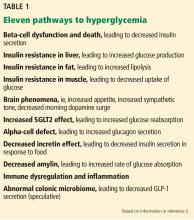
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
KEY POINTS
- At least 11 pathways lead to hyperglycemia; of these, beta-cell dysfunction is central.
- As different classes of diabetes drugs act on different pathways, we can target the pathways contributing to hyperglycemia in the individual patient, using fewer agents and lessening the risk of hypoglycemic episodes.
- In selecting treatment, we should favor drugs that are “gentle” on beta cells, do not cause dangerous hypoglycemia, and improve long-term outcomes as shown in randomized clinical trials.