User login
SAN ANTONIO – About half of women with uterine fibroids became amenorrheic when taking the selective progesterone receptor modulator ulipristal acetate (UPA) during a 12-week study cycle, and women taking UPA experienced significant improvement of quality of life, compared with those taking placebo.
Of those women taking 5 mg of UPA, 40.5%-42% became amenorrheic; of those taking 10 mg, 54.8%-57.3% became amenorrheic, James Liu, MD, reported at the annual meeting of the American Society for Reproductive Medicine. These results compared to amenorrhea rates of 0%-8% for women on placebo (P less than .0001 for all values).
The primary aim of VENUS II was to evaluate UPA’s efficacy and safety as intermittent treatment of abnormal uterine bleeding associated with uterine fibroids. Patients received UPA at either 5 mg or 10 mg orally. Secondary efficacy measures included the maintenance effect of UPA at both doses when compared to placebo, by assessing the rate of amenorrhea and the time to amenorrhea. Another secondary measure assessed uterine fibroid–related quality of life.
Safety was assessed by tracking adverse events through both courses of treatment. The study was not powered to compare the two doses against each other, but rather compared each against placebo.
Altogether, 432 patients were randomized to one of the treatment arms, which was begun after an initial screening period of about 10-12 weeks. The first treatment course lasted 12 weeks, after which patients went off treatment for two menstrual cycles. They then began another treatment course for 12 weeks and were followed for an additional 12 weeks after treatment was stopped.
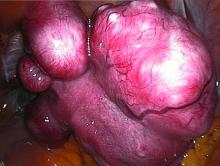
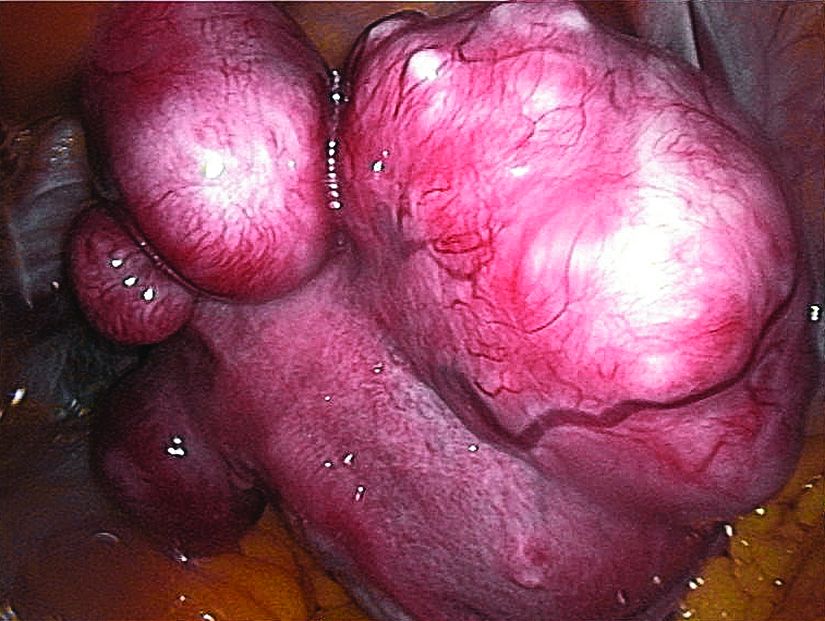
Patients were included if they were premenopausal, aged 18-50 years old, had prolonged bleeding in at least 4 of the last 6 menstrual periods, had menstrual blood loss of at least 80 mL by cycle by the alkaline hematin method, and had at least one discrete leiomyoma without a uterine size greater than 20 weeks. About two-thirds of patients were black, reflecting the higher prevalence of uterine fibroids in this population, said Dr. Liu, professor of medicine and reproductive biology at Case Western Reserve University, Cleveland.
Most patients (60%-80%) had their bleeding controlled on either dose of UPA, compared with fewer than 10% of women taking placebo.
Quality of life data, presented separately at ASRM by Lee Shulman, MD, examined the impact of treatment on patients’ physical and social activities, and also on the severity of symptoms and health-related quality of life.
“The majority of patients on UPA versus a minority of patients on placebo described their menstrual/vaginal bleeding at the end of treatment course 1 as ‘much better’ or ‘very much better,’ ” said Dr. Shulman, chief of clinical genetics in the department of ob.gyn. at Northwestern University in Evanston, Ill.
Of patients taking 5 mg UPA, 75% reported that degree of improvement, as did 87% of those on 10 mg UPA, compared with 17.9% of those taking placebo. .
Adverse events were rare, with hot flashes occurring in about 10% of women taking UPA, compared with less than 2% of those taking placebo. Headaches, fatigue, and nausea were also reported, but rates were not significantly different from rates for those taking placebo. One serious adverse event that was deemed treatment related was a uterine hemorrhage experienced by a woman taking UPA.
“Even in treatment course 1 we already had profound and statistically significant effects in symptoms across the board,” Dr. Shulman said. “How long will they last? We obviously need more data. But the study suggests that the benefits last significantly longer than that associated with leuprolide acetate.”
Ulipristal acetate is already approved by the Food and Drug Administration at a different dosage as emergency contraception. The VENUS II data support its use for women with uterine fibroids, the researchers said. “Our results provide reassurance,” Dr. Liu said. “We can conclude that UPA is effective and safe in the management of uterine fibroids in the U.S. population.”
Dr. Liu reported that he had no relevant disclosures. Dr. Shulman reported relationships with multiple pharmaceutical companies, including Allergan, which funded the VENUS II study.
koakes@frontlinemedcom.com
On Twitter @karioakes
SAN ANTONIO – About half of women with uterine fibroids became amenorrheic when taking the selective progesterone receptor modulator ulipristal acetate (UPA) during a 12-week study cycle, and women taking UPA experienced significant improvement of quality of life, compared with those taking placebo.
Of those women taking 5 mg of UPA, 40.5%-42% became amenorrheic; of those taking 10 mg, 54.8%-57.3% became amenorrheic, James Liu, MD, reported at the annual meeting of the American Society for Reproductive Medicine. These results compared to amenorrhea rates of 0%-8% for women on placebo (P less than .0001 for all values).
The primary aim of VENUS II was to evaluate UPA’s efficacy and safety as intermittent treatment of abnormal uterine bleeding associated with uterine fibroids. Patients received UPA at either 5 mg or 10 mg orally. Secondary efficacy measures included the maintenance effect of UPA at both doses when compared to placebo, by assessing the rate of amenorrhea and the time to amenorrhea. Another secondary measure assessed uterine fibroid–related quality of life.
Safety was assessed by tracking adverse events through both courses of treatment. The study was not powered to compare the two doses against each other, but rather compared each against placebo.
Altogether, 432 patients were randomized to one of the treatment arms, which was begun after an initial screening period of about 10-12 weeks. The first treatment course lasted 12 weeks, after which patients went off treatment for two menstrual cycles. They then began another treatment course for 12 weeks and were followed for an additional 12 weeks after treatment was stopped.
Patients were included if they were premenopausal, aged 18-50 years old, had prolonged bleeding in at least 4 of the last 6 menstrual periods, had menstrual blood loss of at least 80 mL by cycle by the alkaline hematin method, and had at least one discrete leiomyoma without a uterine size greater than 20 weeks. About two-thirds of patients were black, reflecting the higher prevalence of uterine fibroids in this population, said Dr. Liu, professor of medicine and reproductive biology at Case Western Reserve University, Cleveland.
Most patients (60%-80%) had their bleeding controlled on either dose of UPA, compared with fewer than 10% of women taking placebo.
Quality of life data, presented separately at ASRM by Lee Shulman, MD, examined the impact of treatment on patients’ physical and social activities, and also on the severity of symptoms and health-related quality of life.
“The majority of patients on UPA versus a minority of patients on placebo described their menstrual/vaginal bleeding at the end of treatment course 1 as ‘much better’ or ‘very much better,’ ” said Dr. Shulman, chief of clinical genetics in the department of ob.gyn. at Northwestern University in Evanston, Ill.
Of patients taking 5 mg UPA, 75% reported that degree of improvement, as did 87% of those on 10 mg UPA, compared with 17.9% of those taking placebo. .
Adverse events were rare, with hot flashes occurring in about 10% of women taking UPA, compared with less than 2% of those taking placebo. Headaches, fatigue, and nausea were also reported, but rates were not significantly different from rates for those taking placebo. One serious adverse event that was deemed treatment related was a uterine hemorrhage experienced by a woman taking UPA.
“Even in treatment course 1 we already had profound and statistically significant effects in symptoms across the board,” Dr. Shulman said. “How long will they last? We obviously need more data. But the study suggests that the benefits last significantly longer than that associated with leuprolide acetate.”
Ulipristal acetate is already approved by the Food and Drug Administration at a different dosage as emergency contraception. The VENUS II data support its use for women with uterine fibroids, the researchers said. “Our results provide reassurance,” Dr. Liu said. “We can conclude that UPA is effective and safe in the management of uterine fibroids in the U.S. population.”
Dr. Liu reported that he had no relevant disclosures. Dr. Shulman reported relationships with multiple pharmaceutical companies, including Allergan, which funded the VENUS II study.
koakes@frontlinemedcom.com
On Twitter @karioakes
SAN ANTONIO – About half of women with uterine fibroids became amenorrheic when taking the selective progesterone receptor modulator ulipristal acetate (UPA) during a 12-week study cycle, and women taking UPA experienced significant improvement of quality of life, compared with those taking placebo.
Of those women taking 5 mg of UPA, 40.5%-42% became amenorrheic; of those taking 10 mg, 54.8%-57.3% became amenorrheic, James Liu, MD, reported at the annual meeting of the American Society for Reproductive Medicine. These results compared to amenorrhea rates of 0%-8% for women on placebo (P less than .0001 for all values).
The primary aim of VENUS II was to evaluate UPA’s efficacy and safety as intermittent treatment of abnormal uterine bleeding associated with uterine fibroids. Patients received UPA at either 5 mg or 10 mg orally. Secondary efficacy measures included the maintenance effect of UPA at both doses when compared to placebo, by assessing the rate of amenorrhea and the time to amenorrhea. Another secondary measure assessed uterine fibroid–related quality of life.
Safety was assessed by tracking adverse events through both courses of treatment. The study was not powered to compare the two doses against each other, but rather compared each against placebo.
Altogether, 432 patients were randomized to one of the treatment arms, which was begun after an initial screening period of about 10-12 weeks. The first treatment course lasted 12 weeks, after which patients went off treatment for two menstrual cycles. They then began another treatment course for 12 weeks and were followed for an additional 12 weeks after treatment was stopped.
Patients were included if they were premenopausal, aged 18-50 years old, had prolonged bleeding in at least 4 of the last 6 menstrual periods, had menstrual blood loss of at least 80 mL by cycle by the alkaline hematin method, and had at least one discrete leiomyoma without a uterine size greater than 20 weeks. About two-thirds of patients were black, reflecting the higher prevalence of uterine fibroids in this population, said Dr. Liu, professor of medicine and reproductive biology at Case Western Reserve University, Cleveland.
Most patients (60%-80%) had their bleeding controlled on either dose of UPA, compared with fewer than 10% of women taking placebo.
Quality of life data, presented separately at ASRM by Lee Shulman, MD, examined the impact of treatment on patients’ physical and social activities, and also on the severity of symptoms and health-related quality of life.
“The majority of patients on UPA versus a minority of patients on placebo described their menstrual/vaginal bleeding at the end of treatment course 1 as ‘much better’ or ‘very much better,’ ” said Dr. Shulman, chief of clinical genetics in the department of ob.gyn. at Northwestern University in Evanston, Ill.
Of patients taking 5 mg UPA, 75% reported that degree of improvement, as did 87% of those on 10 mg UPA, compared with 17.9% of those taking placebo. .
Adverse events were rare, with hot flashes occurring in about 10% of women taking UPA, compared with less than 2% of those taking placebo. Headaches, fatigue, and nausea were also reported, but rates were not significantly different from rates for those taking placebo. One serious adverse event that was deemed treatment related was a uterine hemorrhage experienced by a woman taking UPA.
“Even in treatment course 1 we already had profound and statistically significant effects in symptoms across the board,” Dr. Shulman said. “How long will they last? We obviously need more data. But the study suggests that the benefits last significantly longer than that associated with leuprolide acetate.”
Ulipristal acetate is already approved by the Food and Drug Administration at a different dosage as emergency contraception. The VENUS II data support its use for women with uterine fibroids, the researchers said. “Our results provide reassurance,” Dr. Liu said. “We can conclude that UPA is effective and safe in the management of uterine fibroids in the U.S. population.”
Dr. Liu reported that he had no relevant disclosures. Dr. Shulman reported relationships with multiple pharmaceutical companies, including Allergan, which funded the VENUS II study.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ASRM 2017
Key clinical point:
Major finding: Between 40.5% and 57.3% of women taking ulipristal acetate (UPA) achieved amenorrhea, compared with 0%-8% of women on placebo (P less than .0001).
Data source: Venus II, a phase 3 prospective, randomized, double-blind, double-dummy, placebo-controlled study that was partially parallel and partially crossover, with 432 patients.
Disclosures: Dr. Liu reported no relevant disclosures; Dr. Shulman reported financial relationships with multiple pharmaceutical companies, including Allergan, which funded the trial.