User login
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
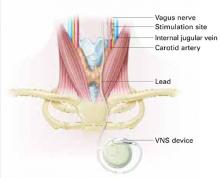
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.