User login
Hepatitis C among the mentally ill: Review and treatment update
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
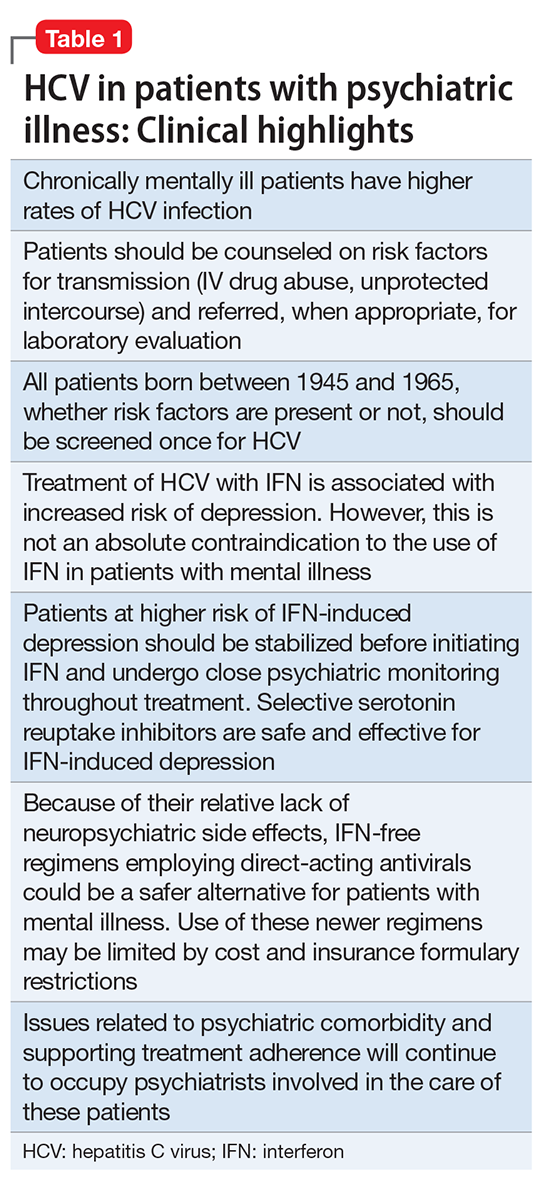
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
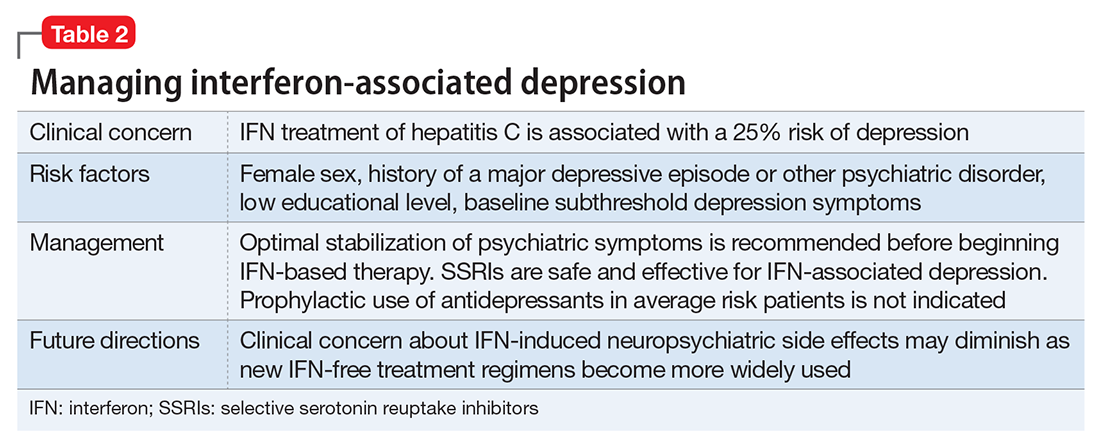
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
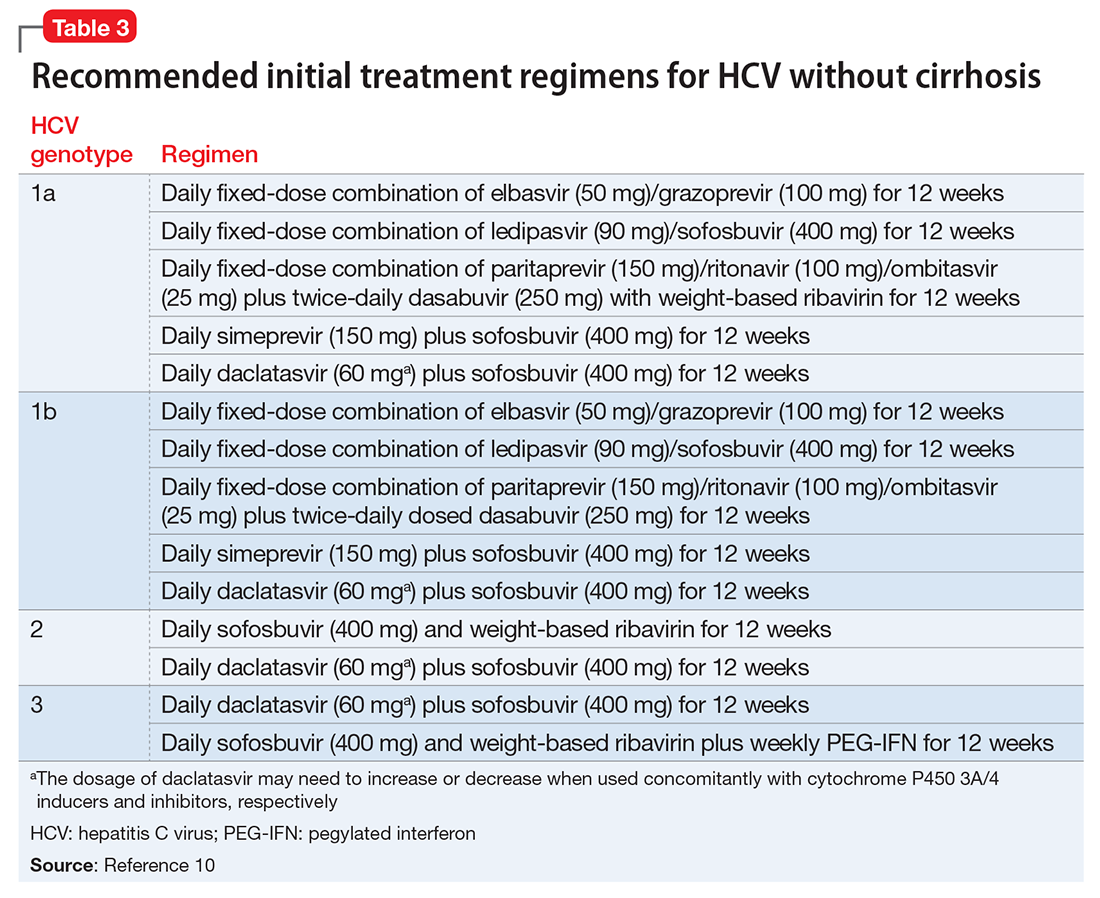
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
Vagus nerve stimulation
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
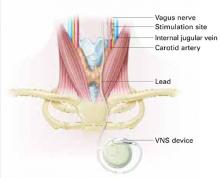
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.