User login
The Diagnosis: Plasmablastic Lymphoma
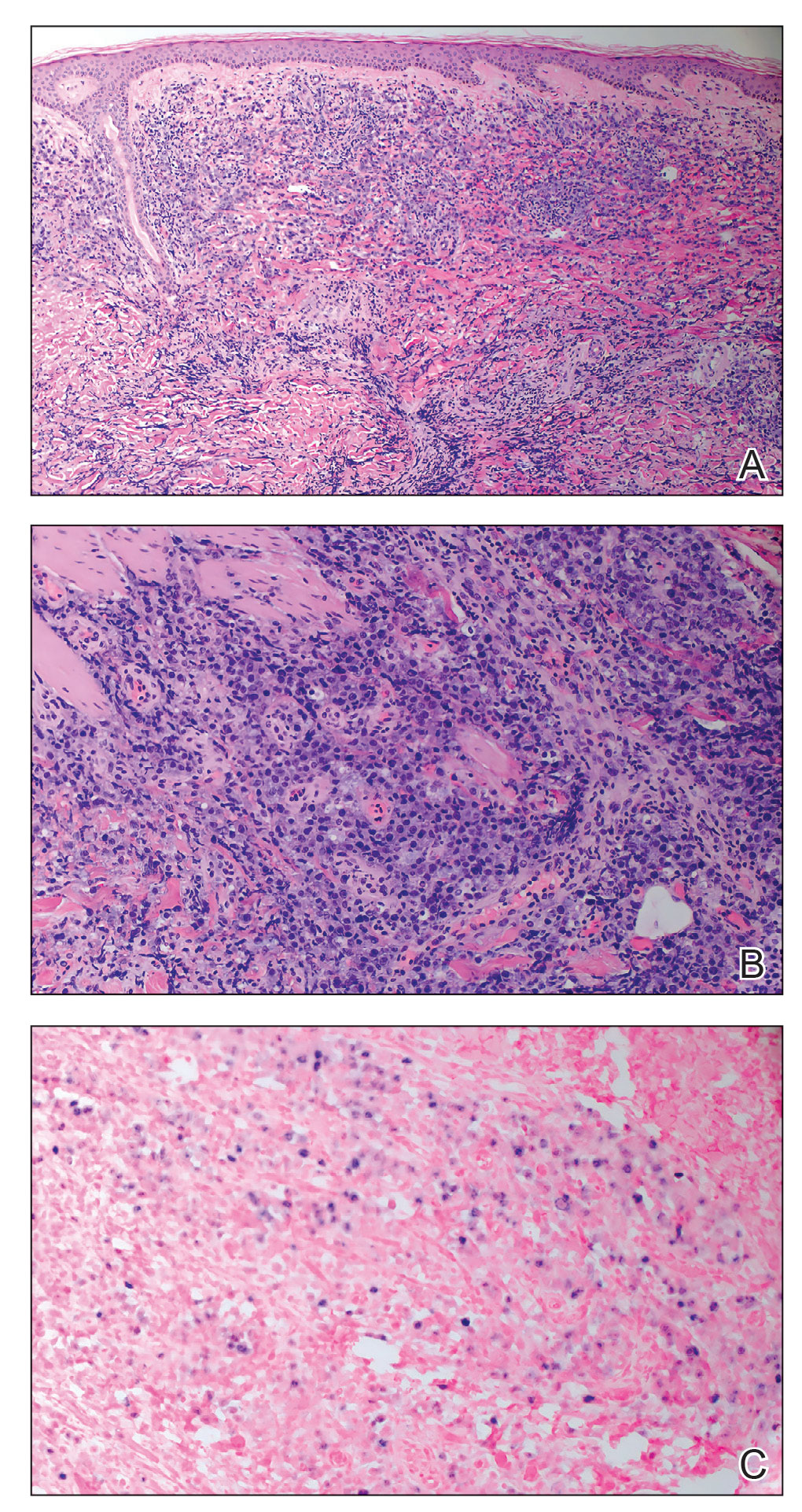
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).
A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
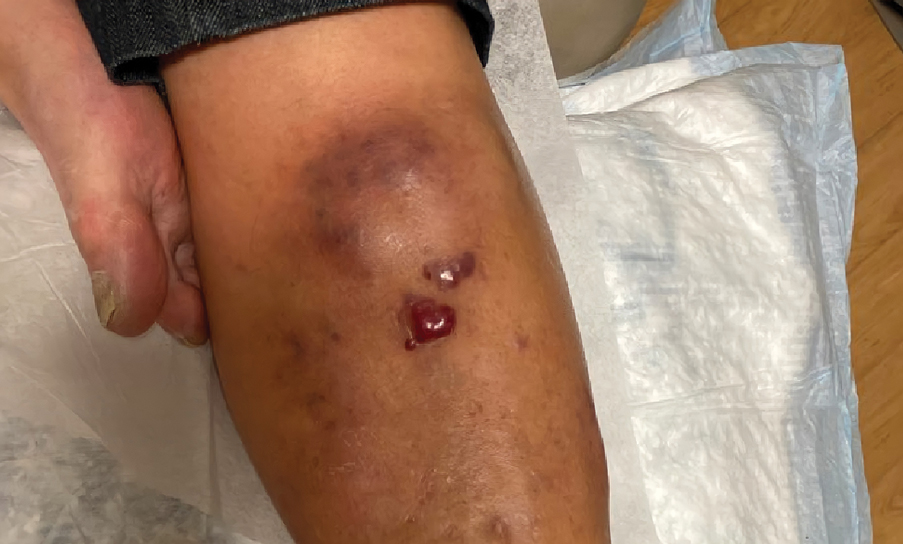
A 67-year-old man with long-standing hepatitis B virus and HIV managed with chronic antiretroviral therapy presented to an urgent care facility with worsening erythema and edema of the legs of 2 weeks’ duration. He was prescribed a 7-day course of cephalexin for presumed cellulitis. Two months later, he developed nodules on the lower extremities. He was seen by podiatry and prescribed a course of amoxicillin–clavulanic acid for presumed infection. Despite 2 courses of antibiotics, his symptoms progressed. The nodules expanded in number and some developed ulceration. Three months into his clinical course, he presented to our dermatology clinic. Physical examination revealed two 2- to 3-cm, violaceous, exophytic, tender nodules. He reported tactile allodynia of the lower extremities and denied fever, chills, night sweats, or weight loss. He also denied exposure to infectious or chemical agents and reported no recent travel. The patient was chronically taking lisinopril/hydrochlorothiazide, escitalopram, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, bupropion, and aspirin with no recent changes. A complete hematologic and biochemical survey largely was unremarkable. His HIV viral load was undetectable with a CD4 count greater than 400/mm3 (reference range, 490–1436/mm3). Lactate dehydrogenase was elevated at 568 IU/L (reference range, 135–225 IU/L). The lower leg lesions were biopsied for confirmatory diagnosis.