User login
BOSTON – The first investigational tau radioligand for PET imaging has identified pathologic tangles in the brain of a man who was confirmed postmortem to have Alzheimer’s disease.
The patient who died was 1 of 12 participants in a study of the agent, [F-18]T808, and his imaging and neuropathologic results show that the agent binds exclusively to tau deposits, Hartmuth Kolb, Ph.D., said at a press conference at the Alzheimer’s Association International Conference 2013.
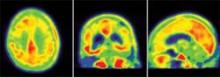
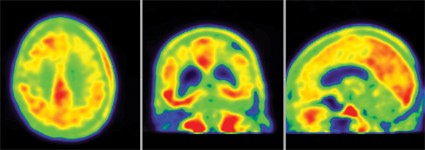
The 85-year-old man died of a pulmonary embolism 2 weeks after his [F-18]T808 scan. His death was unrelated to his diagnosis or to the imaging agent, Dr. Kolb said. Avid Radiopharmaceuticals, the company developing the tracer, obtained the brain for autopsy. The examination showed extensive tau tangles in exactly the same areas identified in a PET scan using [F-18]T808. Tau tangles show as red in the PET images; the warmer the shade, the more dense the tangles.
"The results were quite striking," said Dr. Kolb, senior vice president of research at Avid. "The Braak stage was a 5 or 6, which corresponded with a Mini-Mental State Examination [MMSE] score of 15. On the scan, tau was all over the brain except for a sparing of the sensorimotor cortex, and that was exactly what we had found," in the postmortem exam.
The first human imaging cohort comprised nine patients with diagnosed Alzheimer’s and three healthy control subjects. Investigators compared the in vivo PET images with immunohistochemistry and with staining from fluorescent tau ligand T557, which is used to identify the protein in postmortem exams.
In patients diagnosed with Alzheimer’s, the [F-18]T808 scan showed strong positive correlations with the clinical exam in the temporal lobe, frontal lobe, parietal lobe, and hippocampus. The posterior cingulate gyrus and putamen were relatively spared, and the cerebellum was negative. Patients with an MMSE score of 24 showed moderate tau deposition. Scans of three patients who had an MMSE score of 19, 14, and 3, respectively, showed a progressively denser tau concentration.
There was no significant tau accumulation in any of the three healthy control subjects.
There was one outlier – a patient with an MMSE score of 12 who showed only a very small amount of tau. Dr. Kolb said this patient was probably misdiagnosed as having Alzheimer’s – a problem that continues to plague both clinicians and researchers.
"We don’t have numbers of how many people are misdiagnosed every year, but we do know it’s a big problem," Heather Snyder, Ph.D., director of medical and scientific operations for the Alzheimer’s Association, said in an interview. Misdiagnosis is "not only a mentally difficult thing to go through, but it can potentially keep someone from getting the correct care."
In vivo imaging of Alzheimer’s brain pathology is now held to be an imperative for moving Alzheimer’s research forward. The first agent, Pittsburgh compound B (PiB), which binds to amyloid plaques, has virtually revolutionized research by allowing direct visualization of plaques in living patients. PiB has helped to build a new understanding of prodromal and presymptomatic Alzheimer’s, and it has contributed greatly to patient stratification in clinical trials and to the ability to track therapeutic response.
But PiB is not particularly useful in a clinical setting due to its short, 20-minute half-life. Florbetapir (Amyvid), the investigational PET radioligand florbetaben, and [F-18]T808 all use the same fluorine isotope, making their half-lives 120 minutes and much more friendly for clinical use. [F-18]T808 reaches a plateau of action at about 40 minutes post injection, Dr. Kolb said.
Avid performed its study under an Investigational New Drug designation granted last year, Dr. Kolb said. The company will continue to accrue patients toward an approval request, but it didn’t speculate as to how long that might take, he noted.
[F-18]T808 was being developed by Siemens Medical Solutions USA until last April, when Avid, a subsidiary of Eli Lilly, purchased it along with another investigational tau radiotracer.
The other agent is named [F-18]T807, Lilly spokesperson Eva Catherine Groves said in an interview. She noted that "both have reported similar results and, as such, we plan to complete additional development work on both tracers prior to selecting at least one for advancement into our Alzheimer’s research and development programs."
Dr. Kolb is an employee of Avid Radiopharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – The first investigational tau radioligand for PET imaging has identified pathologic tangles in the brain of a man who was confirmed postmortem to have Alzheimer’s disease.
The patient who died was 1 of 12 participants in a study of the agent, [F-18]T808, and his imaging and neuropathologic results show that the agent binds exclusively to tau deposits, Hartmuth Kolb, Ph.D., said at a press conference at the Alzheimer’s Association International Conference 2013.
The 85-year-old man died of a pulmonary embolism 2 weeks after his [F-18]T808 scan. His death was unrelated to his diagnosis or to the imaging agent, Dr. Kolb said. Avid Radiopharmaceuticals, the company developing the tracer, obtained the brain for autopsy. The examination showed extensive tau tangles in exactly the same areas identified in a PET scan using [F-18]T808. Tau tangles show as red in the PET images; the warmer the shade, the more dense the tangles.
"The results were quite striking," said Dr. Kolb, senior vice president of research at Avid. "The Braak stage was a 5 or 6, which corresponded with a Mini-Mental State Examination [MMSE] score of 15. On the scan, tau was all over the brain except for a sparing of the sensorimotor cortex, and that was exactly what we had found," in the postmortem exam.
The first human imaging cohort comprised nine patients with diagnosed Alzheimer’s and three healthy control subjects. Investigators compared the in vivo PET images with immunohistochemistry and with staining from fluorescent tau ligand T557, which is used to identify the protein in postmortem exams.
In patients diagnosed with Alzheimer’s, the [F-18]T808 scan showed strong positive correlations with the clinical exam in the temporal lobe, frontal lobe, parietal lobe, and hippocampus. The posterior cingulate gyrus and putamen were relatively spared, and the cerebellum was negative. Patients with an MMSE score of 24 showed moderate tau deposition. Scans of three patients who had an MMSE score of 19, 14, and 3, respectively, showed a progressively denser tau concentration.
There was no significant tau accumulation in any of the three healthy control subjects.
There was one outlier – a patient with an MMSE score of 12 who showed only a very small amount of tau. Dr. Kolb said this patient was probably misdiagnosed as having Alzheimer’s – a problem that continues to plague both clinicians and researchers.
"We don’t have numbers of how many people are misdiagnosed every year, but we do know it’s a big problem," Heather Snyder, Ph.D., director of medical and scientific operations for the Alzheimer’s Association, said in an interview. Misdiagnosis is "not only a mentally difficult thing to go through, but it can potentially keep someone from getting the correct care."
In vivo imaging of Alzheimer’s brain pathology is now held to be an imperative for moving Alzheimer’s research forward. The first agent, Pittsburgh compound B (PiB), which binds to amyloid plaques, has virtually revolutionized research by allowing direct visualization of plaques in living patients. PiB has helped to build a new understanding of prodromal and presymptomatic Alzheimer’s, and it has contributed greatly to patient stratification in clinical trials and to the ability to track therapeutic response.
But PiB is not particularly useful in a clinical setting due to its short, 20-minute half-life. Florbetapir (Amyvid), the investigational PET radioligand florbetaben, and [F-18]T808 all use the same fluorine isotope, making their half-lives 120 minutes and much more friendly for clinical use. [F-18]T808 reaches a plateau of action at about 40 minutes post injection, Dr. Kolb said.
Avid performed its study under an Investigational New Drug designation granted last year, Dr. Kolb said. The company will continue to accrue patients toward an approval request, but it didn’t speculate as to how long that might take, he noted.
[F-18]T808 was being developed by Siemens Medical Solutions USA until last April, when Avid, a subsidiary of Eli Lilly, purchased it along with another investigational tau radiotracer.
The other agent is named [F-18]T807, Lilly spokesperson Eva Catherine Groves said in an interview. She noted that "both have reported similar results and, as such, we plan to complete additional development work on both tracers prior to selecting at least one for advancement into our Alzheimer’s research and development programs."
Dr. Kolb is an employee of Avid Radiopharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – The first investigational tau radioligand for PET imaging has identified pathologic tangles in the brain of a man who was confirmed postmortem to have Alzheimer’s disease.
The patient who died was 1 of 12 participants in a study of the agent, [F-18]T808, and his imaging and neuropathologic results show that the agent binds exclusively to tau deposits, Hartmuth Kolb, Ph.D., said at a press conference at the Alzheimer’s Association International Conference 2013.
The 85-year-old man died of a pulmonary embolism 2 weeks after his [F-18]T808 scan. His death was unrelated to his diagnosis or to the imaging agent, Dr. Kolb said. Avid Radiopharmaceuticals, the company developing the tracer, obtained the brain for autopsy. The examination showed extensive tau tangles in exactly the same areas identified in a PET scan using [F-18]T808. Tau tangles show as red in the PET images; the warmer the shade, the more dense the tangles.
"The results were quite striking," said Dr. Kolb, senior vice president of research at Avid. "The Braak stage was a 5 or 6, which corresponded with a Mini-Mental State Examination [MMSE] score of 15. On the scan, tau was all over the brain except for a sparing of the sensorimotor cortex, and that was exactly what we had found," in the postmortem exam.
The first human imaging cohort comprised nine patients with diagnosed Alzheimer’s and three healthy control subjects. Investigators compared the in vivo PET images with immunohistochemistry and with staining from fluorescent tau ligand T557, which is used to identify the protein in postmortem exams.
In patients diagnosed with Alzheimer’s, the [F-18]T808 scan showed strong positive correlations with the clinical exam in the temporal lobe, frontal lobe, parietal lobe, and hippocampus. The posterior cingulate gyrus and putamen were relatively spared, and the cerebellum was negative. Patients with an MMSE score of 24 showed moderate tau deposition. Scans of three patients who had an MMSE score of 19, 14, and 3, respectively, showed a progressively denser tau concentration.
There was no significant tau accumulation in any of the three healthy control subjects.
There was one outlier – a patient with an MMSE score of 12 who showed only a very small amount of tau. Dr. Kolb said this patient was probably misdiagnosed as having Alzheimer’s – a problem that continues to plague both clinicians and researchers.
"We don’t have numbers of how many people are misdiagnosed every year, but we do know it’s a big problem," Heather Snyder, Ph.D., director of medical and scientific operations for the Alzheimer’s Association, said in an interview. Misdiagnosis is "not only a mentally difficult thing to go through, but it can potentially keep someone from getting the correct care."
In vivo imaging of Alzheimer’s brain pathology is now held to be an imperative for moving Alzheimer’s research forward. The first agent, Pittsburgh compound B (PiB), which binds to amyloid plaques, has virtually revolutionized research by allowing direct visualization of plaques in living patients. PiB has helped to build a new understanding of prodromal and presymptomatic Alzheimer’s, and it has contributed greatly to patient stratification in clinical trials and to the ability to track therapeutic response.
But PiB is not particularly useful in a clinical setting due to its short, 20-minute half-life. Florbetapir (Amyvid), the investigational PET radioligand florbetaben, and [F-18]T808 all use the same fluorine isotope, making their half-lives 120 minutes and much more friendly for clinical use. [F-18]T808 reaches a plateau of action at about 40 minutes post injection, Dr. Kolb said.
Avid performed its study under an Investigational New Drug designation granted last year, Dr. Kolb said. The company will continue to accrue patients toward an approval request, but it didn’t speculate as to how long that might take, he noted.
[F-18]T808 was being developed by Siemens Medical Solutions USA until last April, when Avid, a subsidiary of Eli Lilly, purchased it along with another investigational tau radiotracer.
The other agent is named [F-18]T807, Lilly spokesperson Eva Catherine Groves said in an interview. She noted that "both have reported similar results and, as such, we plan to complete additional development work on both tracers prior to selecting at least one for advancement into our Alzheimer’s research and development programs."
Dr. Kolb is an employee of Avid Radiopharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT AAIC 2013
Major finding: The results of in vivo tau tangle PET imaging with [F-18]T808 in a patient diagnosed with Alzheimer’s were later confirmed in a postmortem neuropathologic analysis.
Data source: A study of PET imaging with [F-18]T808 in nine patients diagnosed with Alzheimer’s disease and three healthy controls .
Disclosures: Dr. Kolb is an employee of Avid Radiopharmaceuticals.